I7000
5-Iodo-2′-deoxycytidine
Synonym(s):
5-Iododeoxycytidine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H12IN3O4
CAS Number:
Molecular Weight:
353.11
EC Number:
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
biological source
synthetic (organic)
Assay
≥99% (HPLC)
form
powder
solubility
water: 50 mg/mL, clear, colorless
storage temp.
−20°C
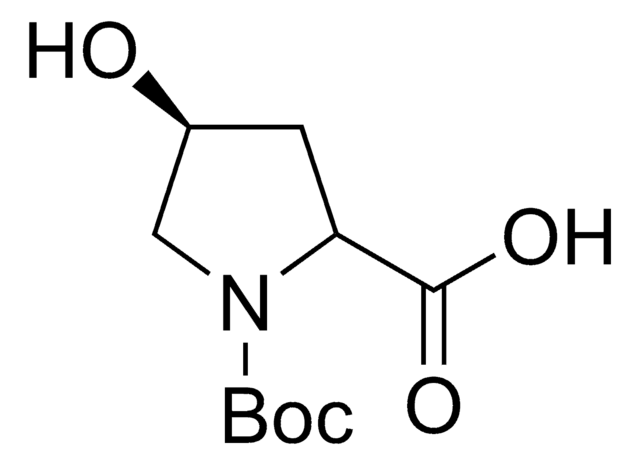
SMILES string
NC1=NC(=O)N(C=C1I)[C@H]2C[C@@H](O)[C@H](CO)O2
InChI
1S/C9H12IN3O4/c10-4-2-13(9(16)12-8(4)11)7-1-5(15)6(3-14)17-7/h2,5-7,14-15H,1,3H2,(H2,11,12,16)/t5?,6?,7-/m1/s1
InChI key
WEVJJMPVVFNAHZ-KPGICGJXSA-N
Looking for similar products? Visit Product Comparison Guide
Application
5-Iodo-2′-deoxycytidine (5-iododeoxycytidine) is used in the construction of DNA oligomers to enable structural studies and photoactivated cross-linking. 5-Iodo-2′-deoxycytidine is used in the synthesis of other modified nucleosides, such as 5-ethynylferrocenyl-2′-deoxycytidine used in semiconductor electrodes and 10-(2-deoxyβ-D-ribofuranosyl)pyrimido[4′,5′:4,5]pyrimido[1,6-a]indole-6,9(7H)-dione (dCPPI).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Masahiro Mizuta et al.
The Journal of organic chemistry, 72(14), 5046-5055 (2007-06-09)
10-(2-Deoxy-beta-D-ribofuranosyl)pyrimido[4',5':4,5]pyrimido[1,6-a]indole-6,9(7H)-dione (dCPPI) and its derivatives were synthesized via the Suzuki-Miyaura coupling reaction of 5-iododeoxycytidine with 5-substituted N-Boc-indole-2-borates and characterized by UV-vis and fluorescence spectroscopy. The new fluorescent nucleosides showed rather large Stokes shifts (116-139 nm) in an aqueous buffer. The
Hereditary orotic aciduria, Lesch-Nyhan syndrome, and xeroderma pigmentosum probed by herpes simplex virus: 125I-iododeoxycytidine incorporation as an assay for viral growth.
J Campisi et al.
Journal of cellular physiology, 114(1), 21-28 (1983-01-01)
Sunil Kumar et al.
Scientific reports, 10(1), 1233-1233 (2020-01-29)
Inferring cell-signaling networks from high-throughput data is a challenging problem in systems biology. Recent advances in cytometric technology enable us to measure the abundance of a large number of proteins at the single-cell level across time. Traditional network reconstruction approaches
Andrew R Pike et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 11(1), 344-353 (2004-11-20)
The ferrocenyl-nucleoside, 5-ethynylferrocenyl-2'-deoxycytidine (1) has been prepared by Pd-catalyzed cross-coupling between ethynylferrocene and 5-iodo-2'-deoxycytidine and incorporated into oligonucleotides by using automated solid-phase synthesis at both silica supports (CPG) and modified single-crystal silicon electrodes. Analysis of DNA oligonucleotides prepared and cleaved
L Fox et al.
Antimicrobial agents and chemotherapy, 23(3), 465-476 (1983-03-01)
The incorporation into DNA of 5-bromocytosine and 5-iodocytosine, derived from their respective administered deoxyribonucleoside analogs, has been demonstrated in studies with cells infected with herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) and in cells transformed with the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![2-[(4-Bromophenyl)methylene]malononitrile](/deepweb/assets/sigmaaldrich/product/structures/581/517/49220e75-b85d-4d94-b647-d741dce149a6/640/49220e75-b85d-4d94-b647-d741dce149a6.png)