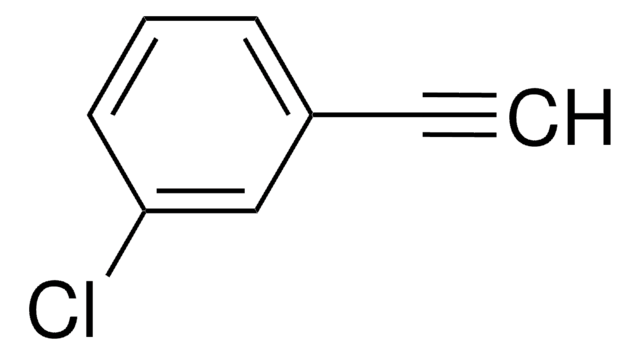
195855
Precocene I
99%
Synonym(s):
7-Methoxy-2,2-dimethyl-3-chromene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H14O2
CAS Number:
Molecular Weight:
190.24
EC Number:
MDL number:
UNSPSC Code:
12352204
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.56 (lit.)
bp
68 °C/0.1 mmHg (lit.)
density
1.052 g/mL at 25 °C (lit.)
application(s)
agriculture
environmental
SMILES string
COc1ccc2C=CC(C)(C)Oc2c1
InChI
1S/C12H14O2/c1-12(2)7-6-9-4-5-10(13-3)8-11(9)14-12/h4-8H,1-3H3
InChI key
CPTJXGLQLVPIGP-UHFFFAOYSA-N
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Fodor et al.
General and comparative endocrinology, 74(1), 18-31 (1989-04-01)
Precocenes (PI and PII) and 114 of their analogs (PAs) were synthetized and tested on C. remanei embryos for their precocene-like (P-like) activities resulting in unusual development at sublethal doses. The P-like activity was quantitated by plotting the probit of
M I Baldellou et al.
Revista espanola de fisiologia, 42(3), 315-317 (1986-09-01)
Antigonadotropic activities of Precocene 1 (P1), Precocene 2 (P2) and Ethoxyprecocene 2 (EP2) on the seed bug Oxycarenus lavaterae (F.) (Heteroptera, Lygaeidae), are reported. EP2 proved to be the most active compound followed by P2 and P1, which agrees with
Angela M Bernard et al.
Organic letters, 4(15), 2565-2567 (2002-07-19)
[reaction: see text] The thionium ion, generated through a cyclopropylcarbinyl-cyclobutyl ring expansion, is, for the first time, intramolecularly intercepted by activated aromatic rings to generate new versatile 2a-methyl-8b-(phenylsulfanyl-1,2a,3,8b-tetrahydro-2H-cyclobuta[c]chromenes.
V Ravindranath et al.
Biochemical pharmacology, 36(4), 441-446 (1987-02-15)
The mechanism of the hepatotoxicity of precocene I has been investigated in male, Sprague-Dawley rats. Administration of a single dose of precocene I caused a large depletion of liver glutathione (GSH) levels that was both time and dose dependent. Concomitant
Atsushi Yaguchi et al.
Journal of agricultural and food chemistry, 57(3), 846-851 (2009-02-05)
Inhibitors of deoxynivalenol production by Fusarium graminearum are useful for protecting crops from deoxynivalenol contamination. We isolated precocenes and piperitone from the essential oils of Matricaria recutita and Eucalyptus dives, respectively, as specific inhibitors of the production of 3-acetyldeoxynivalenol, a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service