P22354
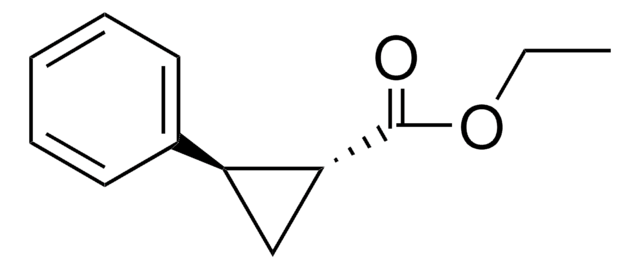
trans-2-Phenylcyclopropane-1-carboxylic acid
95%
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5C3H4CO2H
CAS Number:
Molecular Weight:
162.19
Beilstein:
3197783
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
powder
mp
86-88 °C (lit.)
SMILES string
OC(=O)[C@@H]1C[C@H]1c2ccccc2
InChI
1S/C10H10O2/c11-10(12)9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6H2,(H,11,12)/t8-,9+/m0/s1
InChI key
AHDDRJBFJBDEPW-DTWKUNHWSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
R E Gerkin
Acta crystallographica. Section C, Crystal structure communications, 53 ( Pt 12), 1989-1991 (1998-02-12)
The title compound, (+/-)-cis-2-phenylcyclopropanecarboxylic acid, C10H10O2, crystallized in the centrosymmetric space group P2(1)/n. The hydrogen bonding is of the cyclic dimer type about a center of symmetry; the Odonor...Oacceptor distance is 2.645 (2) A. The carboxylic H atom is ordered
R E Gerkin
Acta crystallographica. Section C, Crystal structure communications, 53 ( Pt 9), 1280-1282 (1997-10-06)
The title compound, C10H10O2, crystallized in space group P2(1) with two molecules in the asymmetric unit. In this structure, hydrogen bonding of the cyclic dimer type [with O ... O 2.623(2)-2.637(2)A] links the two molecules of the asymmetric unit. The
Edmund J Keliher et al.
Organic & biomolecular chemistry, 4(14), 2777-2784 (2006-07-11)
Many carbenoid cyclopropanation reactions promoted by chiral catalysts give product mixtures reflecting impressive diastereo- and enantioselectivities. Few provide a single chiral product efficiently. This limitation has been overcome in cyclopropanations of styrene and isotopically labeled styrenes with alpha-diazoacetates. Convenient syntheses
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service