All Photos(1)
About This Item
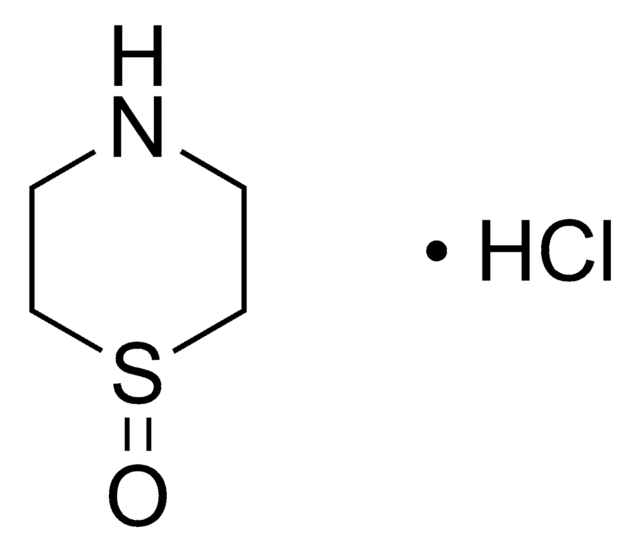
Empirical Formula (Hill Notation):
C4H9NS · HCl
CAS Number:
Molecular Weight:
139.65
Beilstein:
3908104
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98.0% (AT)
form
crystals
mp
174-177 °C
functional group
thioether
storage temp.
2-8°C
SMILES string
Cl[H].C1CSCCN1
InChI
1S/C4H9NS.ClH/c1-3-6-4-2-5-1;/h5H,1-4H2;1H
InChI key
QSJAHJYXDRUZMY-UHFFFAOYSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J I Levin et al.
Bioorganic & medicinal chemistry letters, 16(6), 1605-1609 (2006-01-24)
A series of thiomorpholine sulfonamide hydroxamate TACE inhibitors, all bearing propargylic ether P1' groups, was explored. In particular, compound 5h has excellent in vitro potency against isolated TACE enzyme and in cells, oral activity in a model of TNF-alpha production
[Study of antiradiation drugs. I. Synthesis of some mercapto and amino derivatives of thiomorpholine (author's transl)].
F X Chen et al.
Yao xue xue bao = Acta pharmaceutica Sinica, 15(8), 482-488 (1980-08-01)
B Combourieu et al.
Biodegradation, 9(6), 433-442 (1999-05-21)
Spectrophotometric assays of Mycobacterium aurum MO1 cells extracts gave evidence of a soluble cytochrome P450, involved in the degradative pathway of morpholine, a waste product from the chemical industry. In order to get further information, the kinetics of the biodegradation
I T Ermakova et al.
Mikrobiologiia, 77(5), 617-622 (2008-11-14)
A screening of lignin-degrading basidial fungi that can grow in the presence of thiomorpholine derivatives (the mixture of 1,4-perhydrothiazines) has been performed. Strain Bjerkandera adusta VKM F-3477 was shown to have the maximal rate of growth in the presence of
B Beck et al.
Molecular diversity, 14(3), 479-491 (2010-04-22)
We designed two novel thiolactone scaffolds. Both scaffolds can be accessed by a convergent Ugi multicomponent reaction (MCR) and are, thus, amenable to library synthesis. Design, stereoselectivity, structures, full experimental details, and virtual libraries will be reported.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service