557471
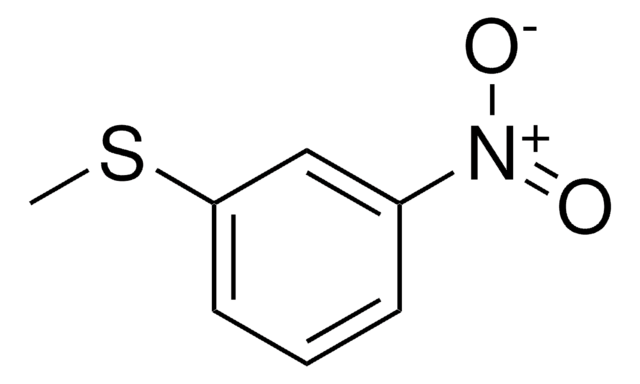
4-Nitrothioanisole
96%
Synonym(s):
Methyl-4-nitrophenyl sulfide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
O2NC6H4SCH3
CAS Number:
Molecular Weight:
169.20
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
solid
mp
66-69 °C (lit.)
68-72 °C (lit.)
SMILES string
CSc1ccc(cc1)[N+]([O-])=O
InChI
1S/C7H7NO2S/c1-11-7-4-2-6(3-5-7)8(9)10/h2-5H,1H3
InChI key
NEZGPRYOJVPJKL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Nitrothioanisole in acetone-d6 solution exhibits twofold barrier to rotation about the Csp2-S bond as 16.1±1.5kJ/mol. 4-Nitrothioanisole undergoes hydrogenation in the presence of sulfided Pd/C catalysts.
Application
4-Nitrothioanisole may be used to synthesize 4-nitrothioanisole sulfoxide and methyl 4-nitrophenyl sulfoxide.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Horseradish peroxidase-catalyzed two-electron oxidations. Oxidation of iodide, thioanisoles, and phenols at distinct sites.
Harris RZ, et al.
The Journal of Biological Chemistry, 268(3), 1637-1645 (1993)
Catalytic hydrogenation of sulfur-containing nitrobenzene over Pd/C catalysts: In situ sulfidation of Pd/C for the preparation of Pd x S y catalysts.
Zhang Q, et al.
Applied Catalysis A: General, 497, 17-21 (2015)
Photoelectrochemical reduction of meta-halonitrobenzenes and related species.
Robert AW and George WJ.
J. Chem. Soc. Perkin Trans. II, 8, 1673-1677 (1995)
The proximate coupling constant, 5 J (H, CH3), and the torsional mobility of the thiomethyl group in some thioanisole derivatives.
Schaefer T, et al.
Canadian Journal of Chemistry, 69(4), 620-624 (1991)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service