Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.51 (lit.)
bp
73-74 °C/47 mmHg (lit.)
density
1.767 g/mL at 25 °C (lit.)
SMILES string
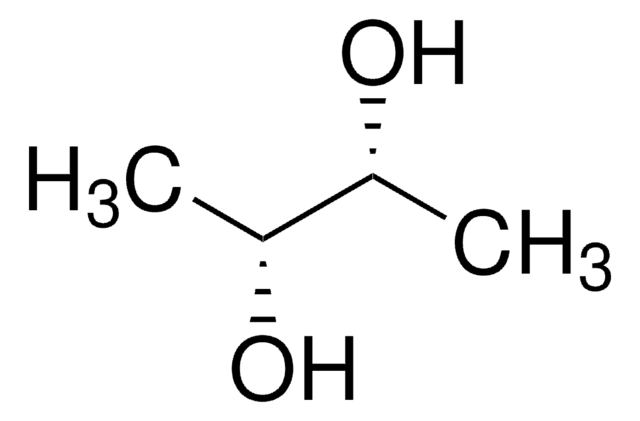
C[C@H](Br)[C@@H](C)Br
InChI
1S/C4H8Br2/c1-3(5)4(2)6/h3-4H,1-2H3/t3-,4+
InChI key
BXXWFOGWXLJPPA-ZXZARUISSA-N
General description
meso-2,3-Dibromobutane is a dihaloalkane. It has two forms of rotational isomers, the trans and gauche isomer. The synthesis and dehalogenation reaction of meso-2,3-dibromobutane has been reported. Its IR and Raman spectra have been investigated. The NMR study of the different conformations of meso-2,3-dibromobutane have been reported.
Application
meso-2,3-Dibromobutane is suitable for use in the synthesis of haloalcohol catalyzed by haloalkane dehalogenases.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
221.0 °F - closed cup
Flash Point(C)
105 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Dehalogenations of 2, 3-dihalobutanes by alkali naphthalenes. CIDNP [chemically induced dynamic nuclear polarization] and stereochemical study.
Garst JF, et al.
Journal of the American Chemical Society, 97(18), 5242-5249 (1975)
Medium effects in rotational isomerism. VI. Inclusion of dipole-dipole interactions in polar solvents.
Abraham RJ.
The Journal of Physical Chemistry, 73(5), 1192-1199 (1969)
Infrared and Raman studies on meso-and (?)-2, 3-dichloro-and-2, 3-dibromo-butanes.
Park PJD and Wyn-Jones E.
J. Chem. Soc. Sect. A, 4222-4426 (1969)
Nuclear magnetic resonance study of rotational isomerism in meso-2, 3-dibromobutane.
Deb KK.
The Journal of Physical Chemistry, 71(9), 3095-3098 (1967)
Haloalkane dehalogenase catalysed desymmetrisation and tandem kinetic resolution for the preparation of chiral haloalcohols.
Westerbeek A, et al.
Tetrahedron, 68(37), 7645-7650 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service