W266418
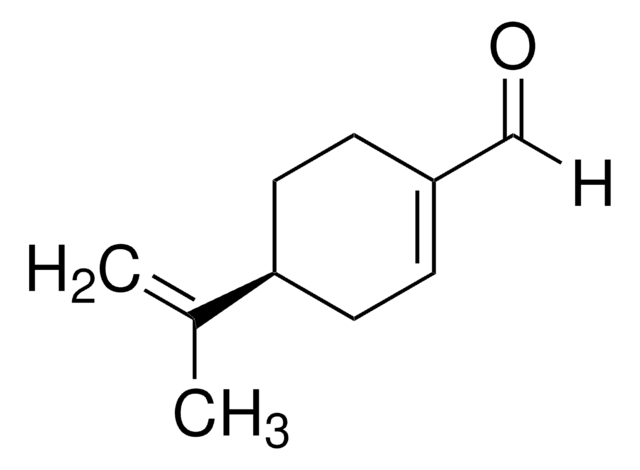
(S)-(−)-Perillyl alcohol
≥95%, FG
Synonym(s):
p-Mentha-1,8-diene-7-ol
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
FG
Halal
Kosher
reg. compliance
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
FDA 21 CFR 172.515
Assay
≥95%
optical activity
[α]20/D −88°, c = 1 in methanol
refractive index
n20/D 1.501 (lit.)
bp
119-121 °C/11 mmHg (lit.)
density
0.96 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
Organoleptic
fatty; green
SMILES string
CC(=C)[C@H]1CCC(CO)=CC1
InChI
1S/C10H16O/c1-8(2)10-5-3-9(7-11)4-6-10/h3,10-11H,1,4-7H2,2H3/t10-/m1/s1
InChI key
NDTYTMIUWGWIMO-SNVBAGLBSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- CYP108N12 initiates p-cymene biodegradation in Rhodococcus globerulus.: This study explores the enzymatic breakdown pathways of monoterpenes, using (S)-(−)-Perillyl alcohol as a precursor, offering insights into microbial degradation processes that could be vital for bioremediation efforts or synthetic biology applications (Giang et al., 2022).
- Orofacial antinociceptive effects of perillyl alcohol associated with codeine and its possible modes of action.: Research demonstrates the pain-relieving properties of (S)-(−)-Perillyl alcohol when combined with codeine, highlighting its potential for developing new analgesic formulations in dental and facial pain management (Limeira et al., 2022).
- Orofacial antinociceptive activity of (S)-(-)-perillyl alcohol in mice: a randomized, controlled and triple-blind study.: This study underpins the effectiveness of (S)-(−)-Perillyl alcohol in reducing orofacial pain in a controlled experimental setup, providing a basis for further clinical trials in pain management (Tomaz-Morais et al., 2017).
- In Vivo Anti-Tumor Activity and Toxicological Evaluations of Perillaldehyde 8,9-Epoxide, a Derivative of Perillyl Alcohol.: Highlights the anti-tumor properties of a novel derivative of (S)-(−)-Perillyl alcohol, suggesting its potential as a therapeutic agent in oncology, with comprehensive studies on its efficacy and safety (Andrade et al., 2016).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service