911666
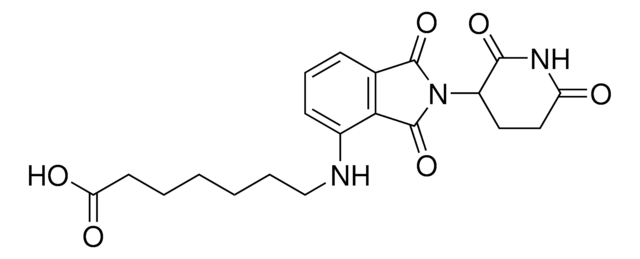
Pomalidomide-C6-NH2 hydrochloride
≥95%
Synonym(s):
4-((6-Aminohexyl)amino)-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione hydrochloride, Crosslinker−E3 ligase ligand conjugate, Pomalidomide conjugate, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader
About This Item
Recommended Products
ligand
pomalidomide
Assay
≥95%
form
powder
reaction suitability
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
functional group
amine
storage temp.
2-8°C
SMILES string
O=C(C(CC1)N(C2=O)C(C3=C2C=CC=C3NCCCCCCN)=O)NC1=O.Cl
Application
Other Notes
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Proteolysis Targeting Chimeras for the Selective Degradation of Mcl-1/Bcl-2 Derived from Nonselective Target Binding Ligands
Chemoselective Synthesis of Lenalidomide-Based PROTAC Library Using Alkylation Reaction
Identification of New Small-Molecule Inducers of Estrogen-related Receptor α (ERRα) Degradation
Discovery of MD-224 as a First-in-Class, Highly Potent and Efficacious PROTAC MDM2 Degrader Capable of Achieving Complete and Durable Tumor Regression
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service