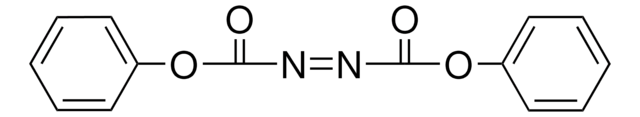680850
Di-(4-chlorobenzyl)azodicarboxylate
97%
Synonym(s):
Bis(4-chlorobenzyl)azodicarboxylate, DCAD
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C16H12Cl2N2O4
CAS Number:
Molecular Weight:
367.18
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
108-112 °C
functional group
azo
chloro
SMILES string
O=C(/N=N\C(OCC1=CC=C(Cl)C=C1)=O)OCC2=CC=C(Cl)C=C2
InChI
1S/C16H12Cl2N2O4/c17-13-5-1-11(2-6-13)9-23-15(21)19-20-16(22)24-10-12-3-7-14(18)8-4-12/h1-8H,9-10H2/b20-19-
InChI key
UIFGGABIJBWRMG-VXPUYCOJSA-N
General description
Learn More at the Professor and Product Portal of Professor Bruce Lipshutz.
Application
Di-(4-chlorobenzyl)azodicarboxylate (DCAD) is a novel, stable, solid alternative to DEAD and DIAD for a variety of Mitsunobu couplings giving a readily separable hydrazine byproduct that can be recycled.
Reactant for preparation of:
- Amino thioesters via guanidine-catalyzed biomimetic enantioselective decarboxylative Mannich and amination reactions of malonic acid half thioesters
- Hydroacylation reaction of aldehydes in Ionic liquid (IL) medium
- DCAD (di-p-chlorobenzyl azodicarboxylate) for Mitsunobu coupling reactions
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
T Amirabadi Farahani et al.
Journal of dairy science, 100(8), 6199-6217 (2017-05-30)
This experiment was conducted to compare conventional (CON; 21 d) and shortened (SH; 10 d) close-up period, and evaluate the effect of shortened close-up period combined with feeding different metabolizable protein (MP) levels on dry matter (DM) intake, metabolic status
R Zimpel et al.
Journal of dairy science, 101(9), 8461-8475 (2018-07-05)
The objective was to determine if the reduction in dry matter (DM) intake of acidogenic diets is mediated by inclusion of acidogenic products, content of salts containing Cl, or changes in acid-base status. The hypothesis was that a decrease in
J E P Santos et al.
Journal of dairy science, 102(3), 2134-2154 (2019-01-08)
The objectives were to use meta-analytic methods to determine the effects of changes in dietary cation-anion difference (DCAD) prepartum on productive performance and health of dairy cows. The literature was systematically reviewed, searching randomized experiments with transition cows that manipulated
Z Wang et al.
Journal of applied microbiology, 130(3), 722-735 (2020-08-07)
The effect of increasing dietary cation-anion difference (DCAD) on rumen fermentation and ruminal microbial community in dairy cows under heat stress (HS) conditions were evaluated. This study was performed as a two-period cross-over design during the summer season, with eight
Bruce H Lipshutz et al.
Organic letters, 8(22), 5069-5072 (2006-10-20)
Di-p-chlorobenzyl azodicarboxylate (DCAD) is introduced as a novel, stable, solid alternative to DEAD and DIAD for a variety of Mitsunobu couplings. DCAD/Ph(3)P-mediated reactions in CH(2)Cl(2) generate a readily separable hydrazine byproduct. [reaction: see text]
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service