657336
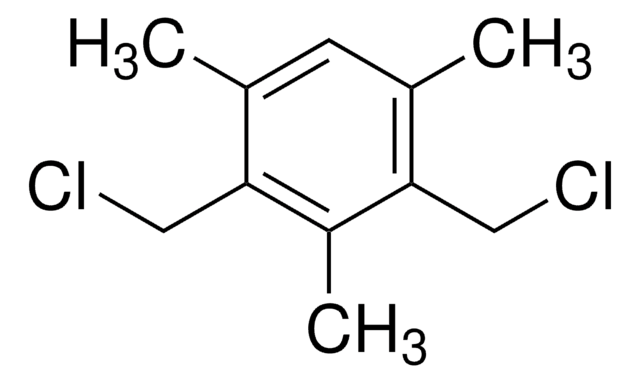
1,3,5-Tris(bromomethyl)benzene
97%
Synonym(s):
2,4,6-Tri(bromomethyl)benzene, Tribromomesitylene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C9H9Br3
CAS Number:
Molecular Weight:
356.88
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
form
solid
mp
94-99 °C
SMILES string
BrCc1cc(CBr)cc(CBr)c1
InChI
1S/C9H9Br3/c10-4-7-1-8(5-11)3-9(2-7)6-12/h1-3H,4-6H2
InChI key
GHITVUOBZBZMND-UHFFFAOYSA-N
General description
1,3,5-Tris(bromomethyl)benzene has three bromo substituents around an aromatic ring that can be used as a cross-linker. It is mainly utilized in the synthesis of ligands and dendrimeric monomers.
Application
1,3,5-Tris(bromomethyl)benzene can be crosslinked with triptycene monomers by using Friedel-Crafts alkylation reaction to form microporous polymers for selective adsorption of CO2 and H2. Proton exchange membranes (PEMs) can be fabricated by covalently linking polybenzimidazole (PBI) and 1,3,5-tris(bromomethyl)benzene as part of the tri-functional bromomethyls for fuel cell applications. It is also used in the synthesis of trifluoroacetamide derivative triaza[33]cyclophane.
A monomer for synthesizing dendrimers and light emitting oligomers.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fabrication of crosslinked polybenzimidazole membranes by trifunctional crosslinkers for high temperature proton exchange membrane fuel cells
Yang J, et al.
International Journal of Hydrogen Energy, 43(6), 3299-3307 (2018)
Synthesis, 39-39 (2008)
Triptycene based microporous polymers (TMPs): Efficient small gas (H2 and CO2) storage and high CO2/N2 selectivity
Bera R, et al.
Microporous and Mesoporous Materials : The Official Journal of the International Zeolite Association, 257(6), 253-261 (2018)
1, 3, 5-Tris (bromomethyl) benzene
Fernandes J, et al.
Acta Crystallographica Section C, Structural Chemistry, 67(6), o198-o200 (2011)
Papri Sutar et al.
Inorganic chemistry, 56(16), 9417-9425 (2017-08-10)
The recent upsurge in research on coordination polymer gels (CPGs) stems from their synthetic modularity, nanoscale processability, and versatile functionalities. Here we report self-assembly of an amphiphilic, tripodal low-molecular weight gelator (L) that consists of 4,4',4-[1,3,5-phenyl-tri(methoxy)]-tris-benzene core and 2,2':6',2″-terpyridyl termini
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service