637386
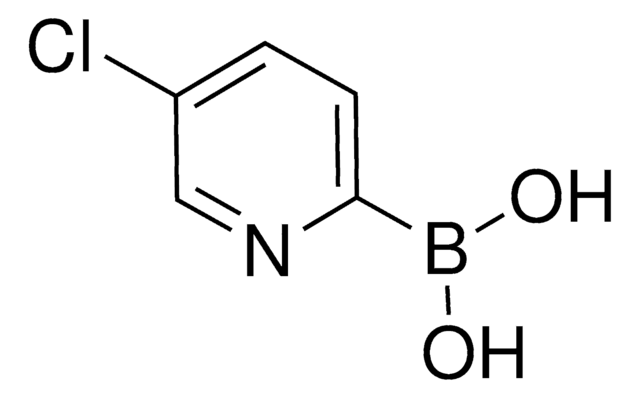
6-Chloro-3-pyridinylboronic acid
≥95.0%
Synonym(s):
2-Chloro-5-pyridineboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C5H5BClNO2
CAS Number:
Molecular Weight:
157.36
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
form
solid
mp
165 °C (lit.)
functional group
chloro
SMILES string
OB(O)c1ccc(Cl)nc1
InChI
1S/C5H5BClNO2/c7-5-2-1-4(3-8-5)6(9)10/h1-3,9-10H
InChI key
WPAPNCXMYWRTTL-UHFFFAOYSA-N
Application
6-Chloro-3-pyridinylboronic acid can be used:
- To prepare biologically significant 3-arylcoumarins by reacting with 3-chlorocoumarin through Suzuki reaction.
- As a substrate in the synthesis of 11-(pyridinylphenyl)steroid with progesterone agonist/antagonist profile.
- As a substrate in the preparation of α- secondary and tertiary pyridines by the reaction of pyridotriazoles with boronic acids.
- As a substrate in the palladium-catalyzed α-arylation of saturated cyclic amines and N-methyl amines.
Other Notes
Contains varying amounts of anhydride
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
α-Arylation of Saturated Azacycles and N-Methylamines via Palladium (II)-catalyzed C (sp3)-H Coupling
Spangler JE, et al.
Journal of the American Chemical Society, 137(37), 11876-11879 (2015)
11-(Pyridinylphenyl) steroids-A new class of mixed-profile progesterone agonists/antagonists
Rewinkel J, et al.
Bioorganic & Medicinal Chemistry, 16(6), 2753-2763 (2008)
Synthesis of 3-arylcoumarins via Suzuki-cross-coupling reactions of 3-chlorocoumarin
Matos MJ, et al.
Tetrahedron Letters, 52(11), 1225-1227 (2011)
Metal-Free Denitrogenative C-C Couplings of Pyridotriazoles with Boronic Acids To Afford α-Secondary and α-Tertiary Pyridines
Dong C, et al.
Organic Letters, 21(11), 4148-4152 (2019)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service