All Photos(1)
About This Item
Linear Formula:
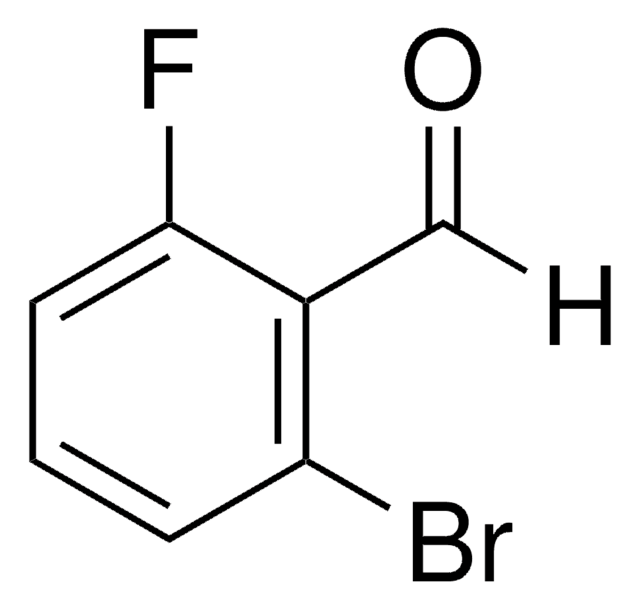
BrC6H4(F)CHO
CAS Number:
Molecular Weight:
203.01
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
mp
51-56 °C (lit.)
storage temp.
2-8°C
SMILES string
Fc1ccc(Br)c(C=O)c1
InChI
1S/C7H4BrFO/c8-7-2-1-6(9)3-5(7)4-10/h1-4H
InChI key
CJUCIKJLMFVWIS-UHFFFAOYSA-N
General description
2-Bromo-5-fluorobenzaldehyde can be prepared by reacting 2-bromo-5-fluorotoluene with N-bromosuccinimide. Its crystals exhibit monoclinic crystal system and space group P21/c.
Application
2-Bromo-5-fluorobenzaldehyde may be used in the synthesis of dihydrobenzoxaboroles bearing aryl, heteroaryl or vinyl substituents at the 1-position and 5-fluoro-1,3-dihydro-1-hydroxy-2,1-benzoxaborole.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
No data available
Flash Point(C)
No data available
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Izabela D Madura et al.
Acta crystallographica. Section E, Structure reports online, 67(Pt 2), o414-o415 (2011-04-28)
In the crystal structure of the title compound, C(7)H(6)BFO(2), a broad-spectrum anti-fungal drug (AN2690), the planar [maximum deviation 0.035 (1) Å] mol-ecules form centrosymmetric R(2) (2)(8) dimers via strong O-H⋯O hydrogen bonds. The dimers are arranged into layers by weak inter-molecular C-H⋯O
2-Bromo-5-fluorobenzaldehyde.
Tureski RE and Tanski JM.
Acta Crystallographica Section E, Structure Reports Online, 69(8), o1246-o1246 (2013)
Nuclear spin-spin coupling via nonbonded interactions. 4. Fluorine-fluorine and hydrogen-fluorine coupling in substituted benzo [c] phenanthrenes.
Mallory FB, et al.
The Journal of Organic Chemistry, 50(4), 457-461 (1985)
Stephen J Baker et al.
Journal of medicinal chemistry, 49(15), 4447-4450 (2006-07-21)
A structure-activity relationship investigation for a more efficacious therapy to treat onychomycosis, a fungal infection of the toe and fingernails, led to the discovery of a boron-containing small molecule, 5-fluoro-1,3-dihydro-1-hydroxy-2,1-benzoxaborole (AN2690), which is currently in clinical trials for onychomycosis topical
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service