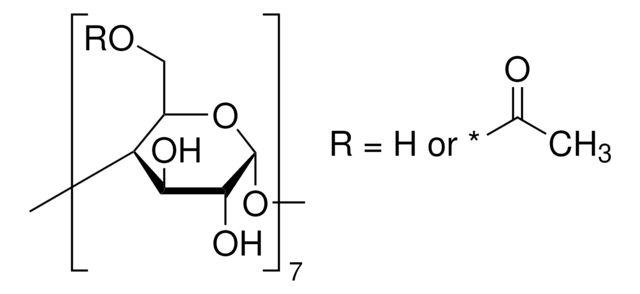
390690
(2-Hydroxypropyl)-α-cyclodextrin
average Mw ~1,180
Synonym(s):
α‐HPCD, 2OHpαCD, 2‐hydroxypropyl‐α‐CD, HPαCD, HP-α-CD
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
form
powder
optical activity
[α]20/D +125°, c = 1 in H2O
mol wt
average Mw ~1,180
extent of labeling
0.6 molar substitution
mp
245 °C (dec.) (lit.)
Looking for similar products? Visit Product Comparison Guide
General description
(2-Hydroxypropyl)-α-cyclodextrin is a hydroxyalkyl derivative of cyclodextrin, which is generally used as a substitute of α-cyclodextrin for potential application in food, cosmetics and pharmaceutical industries. It may be used as a parenteral drug carrier on account of its attractive characteristics such as: a greater extent of solubility in water, high dissolution capacity for weakly soluble analytes, and low toxicity.
Application
- (2-Hydroxypropyl)-α-cyclodextrin can be used as a chiral selector to resolve tryptophan racemate by capillary electrophoresis.
- It can be used to synthesize biodegradable polyrotaxanes for fabricating blood-contacting devices.
- Co-polymer based on (2-hydroxypropyl)-α-cyclodextrin cross-linked with polyethylenimine can be used as a gene delivery vector.
- It can also be used as stabilizer for antibacterial iodine formulations, as it forms a stable inclusion complex with iodine and improves aqueous solubility.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A novel co-polymer based on hydroxypropyl α-cyclodextrin conjugated to low molecular weight polyethylenimine as an in vitro gene delivery vector.
Huang H, et al.
International Journal of Molecular Sciences, 9(11), 2278-2289 (2008)
Effect of biodegradable polyrotaxanes on platelet activation.
Yui N, et al.
Bioconjugate Chemistry, 9(1), 118-125 (1998)
Interaction of iodine with 2-hydroxypropyl-α-cyclodextrin and its bactericidal activity.
Tomono K, et al.
Drug Development and Industrial Pharmacy, 28(10), 1303-1309 (2002)
2-Hydroxypropylated cyclodextrins as a sustained-release carrier for fragrance materials
TANAKA M, et al.
Chemical & Pharmaceutical Bulletin, 44(2), 416-420 (1996)
Recognition mechanism of d-and l-tryptophan enantiomers using 2-hydroxypropyl-α-or β-cyclodextrins as chiral selectors.
Malta LFB, et al.
Tetrahedron Asymmetry, 19(10), 1182-1188 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service