176893
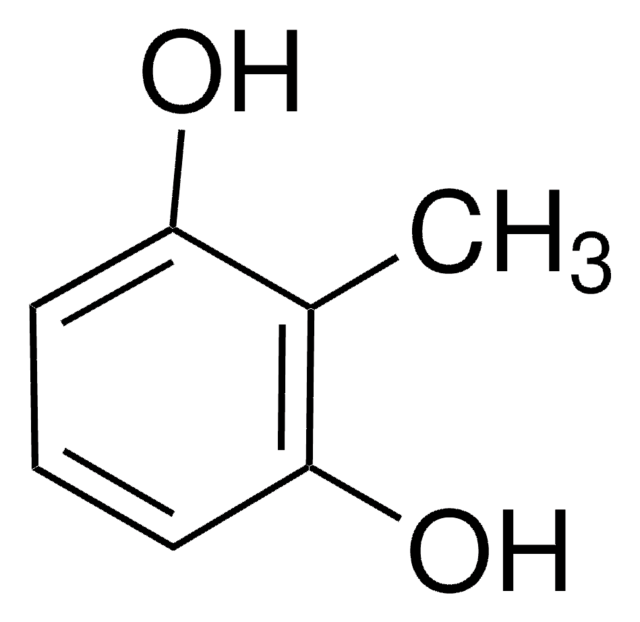
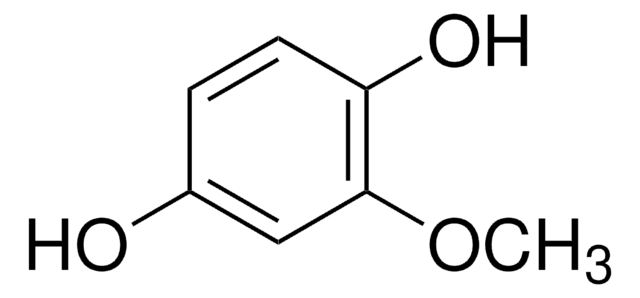
2-Methoxyhydroquinone
98%
Synonym(s):
2,5-Dihydroxyanisol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3O)C6H3(OH)2
CAS Number:
Molecular Weight:
140.14
Beilstein:
2045285
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
98%
form
solid
mp
88-91 °C (lit.)
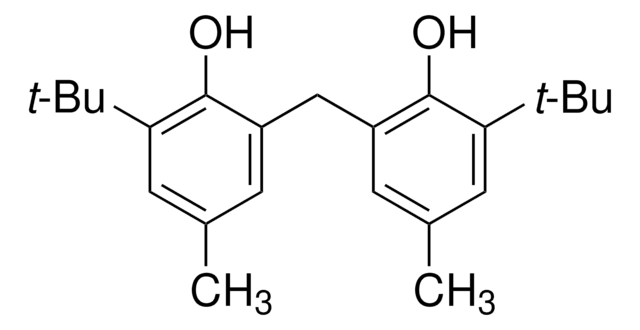
SMILES string
COc1cc(O)ccc1O
InChI
1S/C7H8O3/c1-10-7-4-5(8)2-3-6(7)9/h2-4,8-9H,1H3
InChI key
LAQYHRQFABOIFD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shou Ito et al.
Biotechnology for biofuels, 13, 18-18 (2020-02-06)
Vanillin is the main byproduct of alkaline-pretreated lignocellulosic biomass during the process of fermentable-sugar production and a potent inhibitor of ethanol production by yeast. Yeast cells are usually exposed to vanillin during the industrial production of bioethanol from lignocellulosic biomass.
David Sillam-Dussès et al.
PloS one, 7(10), e46431-e46431 (2012-10-17)
Labial glands are present in all castes and developmental stages of all termite species. In workers, their secretion contains a food-marking pheromone and digestive enzymes, while soldier secretion plays a defensive role. However, these functions were studied only in a
Maher A Qaddoura et al.
International journal of molecular sciences, 10(11), 4772-4788 (2010-01-21)
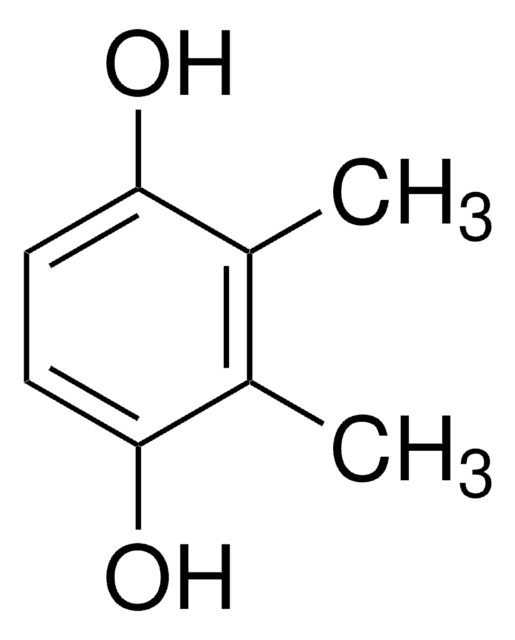
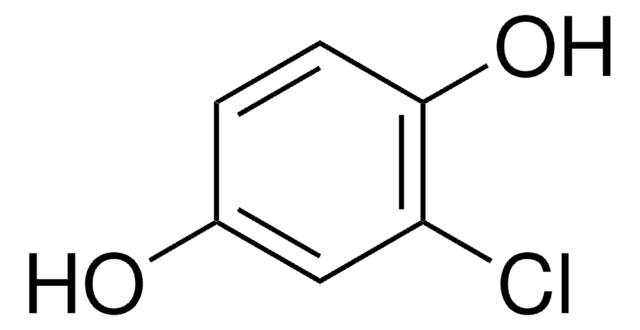
Several divinylic mesogenic monomers were synthesized based on coupling the monomer 4-(4-pentenyloxy)benzoic acid with chlorohydroquinone, 2,5-dihydroxy- acetophenone, methylhydroquinone or 2-methoxyhydroquinone. This resulted in novel mesogens of phenylene esters with different lateral substituent groups. The effect of the lateral substituent group
Koorosh Ashrafi et al.
International journal of pharmaceutics, 524(1-2), 226-237 (2017-04-05)
Drug release from chemoembolization microspheres stimulated by the presence of a chemically reducing environment may provide benefits for targeting drug resistant and metastatic hypoxic tumours. A water-soluble disulfide-based bifunctional cross-linker bis(acryloyl)-(l)-cystine (BALC) was synthesised, characterised and incorporated into a modified
Denilson F Oliveira et al.
Experimental parasitology, 199, 17-23 (2019-02-23)
Exposing second-stage juveniles (J2) of Meloidogyne incognita in vitro to a phenolic compound sometimes fails to cause J2 mortality, but in tests in vivo the same compound may reduce the infectivity and population of the nematode. This work aimed to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service