146188
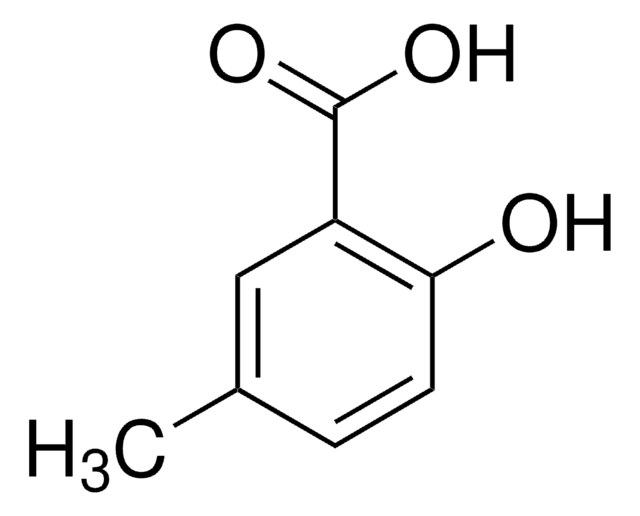
2-Hydroxy-5-methoxybenzoic acid
98%
Synonym(s):
5-Methoxysalicylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3OC6H3(OH)CO2H
CAS Number:
Molecular Weight:
168.15
Beilstein:
2209647
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
mp
141-143 °C (lit.)
functional group
carboxylic acid
SMILES string
COc1ccc(O)c(c1)C(O)=O
InChI
1S/C8H8O4/c1-12-5-2-3-7(9)6(4-5)8(10)11/h2-4,9H,1H3,(H,10,11)
InChI key
IZZIWIAOVZOBLF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Hydroxy-5-methoxybenzoic acid is matrix additive which enhances the electrical conductivity of the matrix crystal during matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS).
Application
2-Hydroxy-5-methoxybenzoic acid was used to evaluate MALDI matrix/solvent combinations for intact spore mass spectrometry of Fusarium species.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kathrin Stavenhagen et al.
Molecular & cellular proteomics : MCP, 17(6), 1225-1238 (2017-12-14)
Human C1-inhibitor (C1-Inh) is a serine protease inhibitor and the major regulator of the contact activation pathway as well as the classical and lectin complement pathways. It is known to be a highly glycosylated plasma glycoprotein. However, both the structural
Stephanie Holst et al.
Scientific reports, 7(1), 16623-16623 (2017-12-02)
To characterise pancreatic cancer cells from different sources which are used as model systems to study the metastatic behaviour in pancreatic ductal adenocarcinoma (PDAC), we compared the N-glycan imprint of four PDAC cells which were previously shown to differ in
Lise Hafkenscheid et al.
Molecular & cellular proteomics : MCP, 16(2), 278-287 (2016-12-14)
Recently, we showed the unexpectedly high abundance of N-linked glycans on the Fab-domain of Anti-Citrullinated Protein Antibodies (ACPA). As N-linked glycans can mediate a variety of biological functions, we now aimed at investigating the structural composition of the Fab-glycans of
T Nishihata et al.
Journal of pharmacobio-dynamics, 7(5), 278-285 (1984-05-01)
The rectal absorption of pepleomycin sulfate (PEPS) in rats was increased significantly by the coadministration with each of diclofenac (DC), sodium 5-methoxysalicylate (5-MSA) and phenylalanine enamine of ethylacetoacetate (Enamine). 5-MSA increased the lymphatic uptake of PEPS after rectal administration while
Jasmin Kemptner et al.
Rapid communications in mass spectrometry : RCM, 23(6), 877-884 (2009-02-19)
Unambiguous identification of mycotoxin-producing fungal species as Fusarium is of great relevance to agriculture and the food-producing industry as well as in medicine. Protein profiles of intact fungal spores, such as Penicillium, Aspergillus and Trichoderma, derived from matrix-assisted laser desorption/ionization
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service