668540
(2S,5S)-(–)-5-Benzyl-3-methyl-2-(5-methyl-2-furyl)-4-imidazolidinone
95%
Synonym(s):
(2S,5S)-3-Methyl-2-(5-methyl-2-furanyl)-5-(phenylmethyl)-4-imidazolidinone
About This Item
Recommended Products
Assay
95%
refractive index
n20/D 1.5598
functional group
phenyl
SMILES string
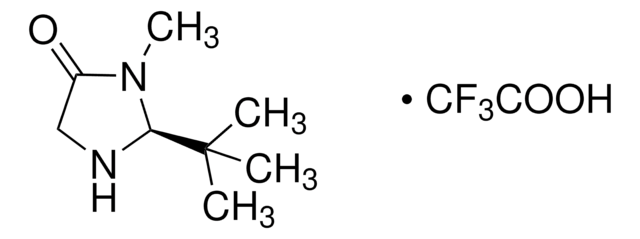
CN1[C@H](N[C@@H](Cc2ccccc2)C1=O)c3ccc(C)o3
InChI
1S/C16H18N2O2/c1-11-8-9-14(20-11)15-17-13(16(19)18(15)2)10-12-6-4-3-5-7-12/h3-9,13,15,17H,10H2,1-2H3/t13-,15-/m0/s1
InChI key
IQIMPHPFMHVWBZ-ZFWWWQNUSA-N
Related Categories
Application
- Enantioselective organocatalytic transfer hydrogenation of cycloalkenones with tert-Bu Hantzsch ester as source of hydrogen
- Alkylation of indoles with an α,β-disubstituted α,β-unsaturated aldehyde in the enantioselective preparation of a selective serotonin reuptake inhibitor
- MacMillan reaction
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
>230.0 °F
Flash Point(C)
> 110 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Discover Professor David MacMillan's groundbreaking metal-free asymmetric catalysis using imidazolidinone-based organocatalysts for versatile transformations.
Discover Professor David MacMillan's groundbreaking metal-free asymmetric catalysis using imidazolidinone-based organocatalysts for versatile transformations.
Discover Professor David MacMillan's groundbreaking metal-free asymmetric catalysis using imidazolidinone-based organocatalysts for versatile transformations.
Discover Professor David MacMillan's groundbreaking metal-free asymmetric catalysis using imidazolidinone-based organocatalysts for versatile transformations.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![(S)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol trimethylsilyl ether 97%](/deepweb/assets/sigmaaldrich/product/structures/396/398/09a397b1-b5f5-420f-98da-adf9017cef56/640/09a397b1-b5f5-420f-98da-adf9017cef56.png)



