All Photos(2)
About This Item
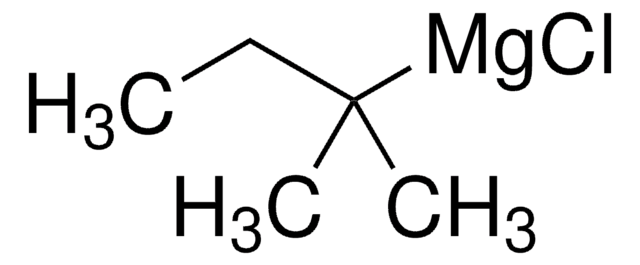
Empirical Formula (Hill Notation):
C8H7Br
CAS Number:
Molecular Weight:
183.05
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
refractive index
n20/D 1.59 (lit.)
bp
90 °C/10.5 mmHg (lit.)
density
1.45 g/mL at 25 °C (lit.)
functional group
bromo
SMILES string
BrC1Cc2ccccc12
InChI
1S/C8H7Br/c9-8-5-6-3-1-2-4-7(6)8/h1-4,8H,5H2
InChI key
AYNXHFRDABNHRX-UHFFFAOYSA-N
Related Categories
General description
1-Bromobenzocyclobutene is a useful synthon. It has important applications in organometallic methodology. Reaction between cycloheptatriene, bromoform, potassium carbonate and 18-crown-6 at 140°C yields 1-bromobenzocyclobutene.
Application
1-Bromobenzocyclobutene may be used in the synthesis of following compounds:
- five-membered zirconacycles
- benzocyclobutenol and benzocyclobutenone
- 2,3-dimethoxyprotoberberinium bromide, via reaction with 3,4-dihydro-6,7-dimethoxyisoquinoline
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
204.8 °F - closed cup
Flash Point(C)
96.00 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
1-Bromobenzocyclobutene: a convenient entry into the benzocyclobutene ring system.
DeCamp MR and Viscogliosi LA.
The Journal of Organic Chemistry, 46(19), 3918-3920 (1981)
Studies on the syntheses of heterocyclic compounds-DXLV: An alternative synthesis of the protoberberine ring system.
Kametani T, et al.
Tetrahedron, 30(9), 1043-1046 (1974)
Condensed Cyclobutane Aromatic Compounds. IX. Benzocyclobutenol and Benzocyclobutenone.
Cava MP and Muth K.
Journal of the American Chemical Society, 82(3), 652-654 (1960)
T V V Ramakrishna et al.
Organic letters, 5(6), 877-879 (2003-03-14)
[reaction: see text] Commercially available 1-bromobenzocyclobutene is a potentially useful synthon particularly with the application of organometallic methodology. Here we show that it is readily converted into Cp(2)Zr(benzocyclobutadiene), which couples with alkynes or nitriles giving five-membered zirconacycles. Treatment of these
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service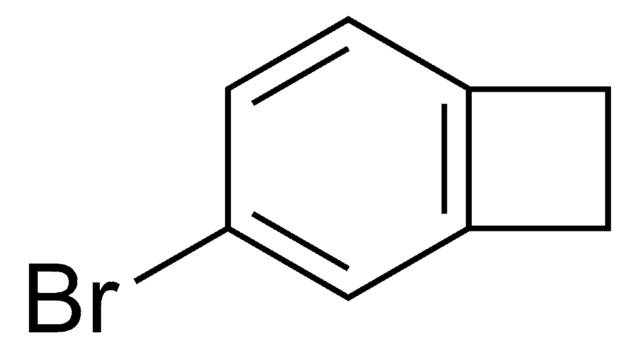
![BICYCLO[4.2.0]OCTA-1,3,5-TRIEN-7-ONE AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/105/021/61bb8f7c-e86a-48bf-8d63-aa68ad3c21df/640/61bb8f7c-e86a-48bf-8d63-aa68ad3c21df.png)