467464
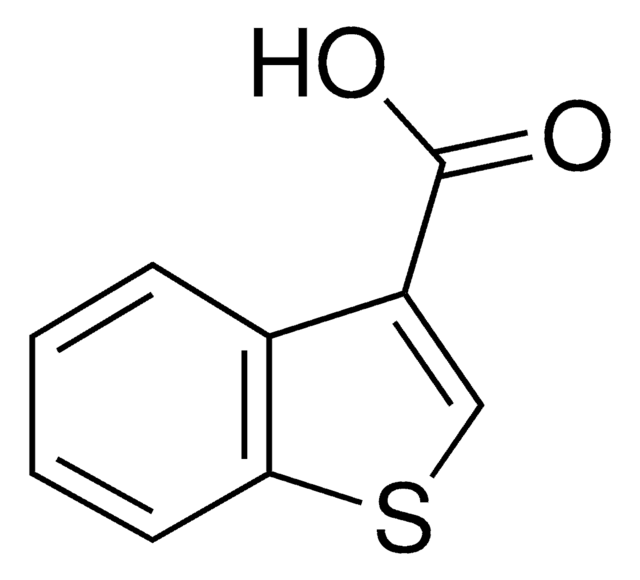
Thianaphthene-2-carboxylic acid
98%
Synonym(s):
Benzo[b]thiophene-2-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H6O2S
CAS Number:
Molecular Weight:
178.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
241-244 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)c1cc2ccccc2s1
InChI
1S/C9H6O2S/c10-9(11)8-5-6-3-1-2-4-7(6)12-8/h1-5H,(H,10,11)
InChI key
DYSJMQABFPKAQM-UHFFFAOYSA-N
General description
Thianaphthene-2-carboxylic acid, a benzothiophene, is a heterocyclic sulfur compound. It undergoes degradation (23%) by employing a mixture of washed cells of Rhodococcus erythropolis DS-3 and Gordonia sp. C-6.
Application
Thianaphthene-2-carboxylic acid may be used for the fabrication of carboxylated conducting polymer/CNTs (carbon nanotubes) composites thin films.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J C Robin et al.
Journal of medicine, 11(1), 15-27 (1980-01-01)
Total skeletal calcium was determined in female mice with the aid of whole body neutron activation analysis. Three months treatment with heaprin produced significant osteoporosis in C3-H/St(Ha) mice but not in C57/BL6(J) mice. This was more pronounced in the younger
J C Robin et al.
Calcified tissue international, 36(2), 194-199 (1984-03-01)
The purpose of the present study was to investigate the mechanism of action on bone of Benzo(B)Thiophene-2-Carboxylic Acid (BL-5583). BL-5583, at a dose range of 0.01-100 micrograms/ml, inhibited spontaneous as well as A23187 and PTH-induced bone resorption in tissue culture.
The synthesis of some substituted thianaphthene-2-carboxamides and their antifungal properties.
Goettsch RW and Wiese GA.
Journal of the American Pharmaceutical Association, 47(5), 319-322 (1958)
Guo-Qiang Li et al.
Biotechnology letters, 30(10), 1759-1764 (2008-06-03)
Substituted benzothiophenes (BTs) and dibenzothiophenes (DBTs) remain in diesel oil following conventional desulfurization by hydrodesulfurization. A mixture of washed cells (13.6 g dry cell wt l(-1)) of Rhodococcus erythropolis DS-3 and Gordonia sp. C-6 were employed to desulfurize hydrodesulfurized diesel
Fabrication of Carboxylated Conducting Polymer/CNTs Composites Thin Films for Immunosensor Application.
Netsuwan P, et al.
Mol. Cryst. Liq. Cryst., 580(1), 7-14 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![Methyl benzo[b]thiophene-2-carboxylate 97%](/deepweb/assets/sigmaaldrich/product/structures/216/435/32affee2-b18f-4b51-a4fc-fd77f1af231c/640/32affee2-b18f-4b51-a4fc-fd77f1af231c.png)






![Benzo[b]thiophene-2-carboxaldehyde 97%](/deepweb/assets/sigmaaldrich/product/structures/321/060/32405a4e-5720-4c6d-91cf-115c747270c4/640/32405a4e-5720-4c6d-91cf-115c747270c4.png)
