All Photos(1)
About This Item
Linear Formula:
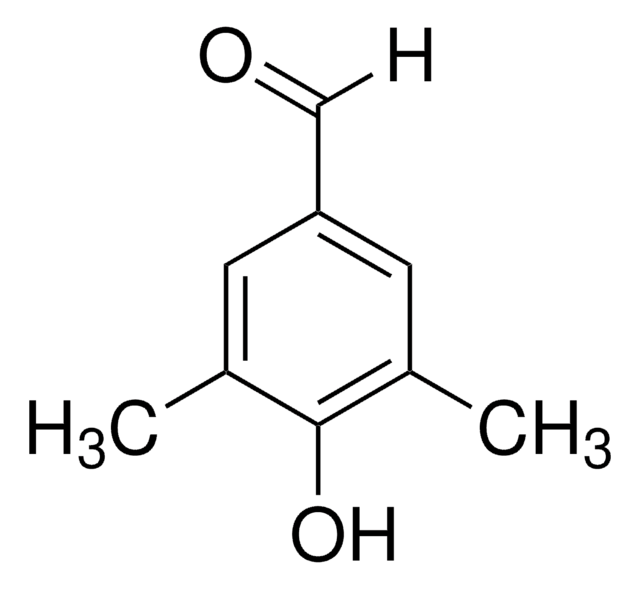
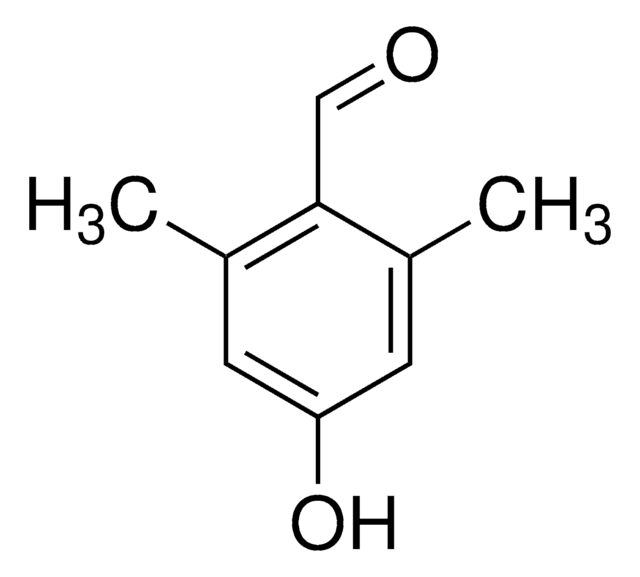
HOC6H2(OCH3)2CHO
CAS Number:
Molecular Weight:
182.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
mp
225 °C (dec.) (lit.)
functional group
aldehyde
SMILES string
COc1cc(O)cc(OC)c1C=O
InChI
1S/C9H10O4/c1-12-8-3-6(11)4-9(13-2)7(8)5-10/h3-5,11H,1-2H3
InChI key
HZWPJAZIRZFCGX-UHFFFAOYSA-N
General description
2,6-Dimethoxy-4-hydroxybenzaldehyde (4-hydroxy-2,6-dimethoxybenzaldehyde) is a p-hydroxybenzaldehyde derivative. Its synthesis by Vielsmeyer-Haack reaction and confirmation of the product formation by 1H NMR has been reported. Its structure has been investigated. The sodium salt of 4-hydroxy-2,6-dimethoxybenzaldehyde may be used to derivatize Merrifield resin to form resin-bound aldehyde.
Application
2,6-Dimethoxy-4-hydroxybenzaldehyde (4-hydroxy-2,6-dimethoxybenzaldehyde) may be used as a test compound to investigate the bactericidal activity of benzaldehydes against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes and Salmonella enterica.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mendel Friedman et al.
Journal of food protection, 66(10), 1811-1821 (2003-10-24)
We evaluated the bactericidal activities of 35 benzaldehydes, 34 benzoic acids, and 1 benzoic acid methyl ester against Campylobacter jejuni, Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella enterica when these compounds were substituted on the benzene ring with 0, 1
A Structure-Based Activation Model of Phenol-Receptor Protein Interactions.
Lee K.
Bull. Korean Chem. Soc., 18, 16-22 (1997)
Ming-Fu Cheng et al.
Journal of combinatorial chemistry, 6(1), 99-104 (2004-01-13)
A library of 1,4-benzodiazepine-2,5-dione dicarboxylate derivatives containing aryl substituents at N(1)- and N(4)-positions to mimic the amino acid residues of Try-13, Phe-14, and Asp-18 in endothelin-1 is established by using the starting materials of alpha-amino esters, hydroxybenzaldehydes, nitrobenzoyl chlorides, and
4-Hydroxy-2, 6-dimethoxybenzaldehyde.
Wuyts C, et al.
Acta Crystallographica Section E, Structure Reports Online, 61(1), 79-80 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service