377619
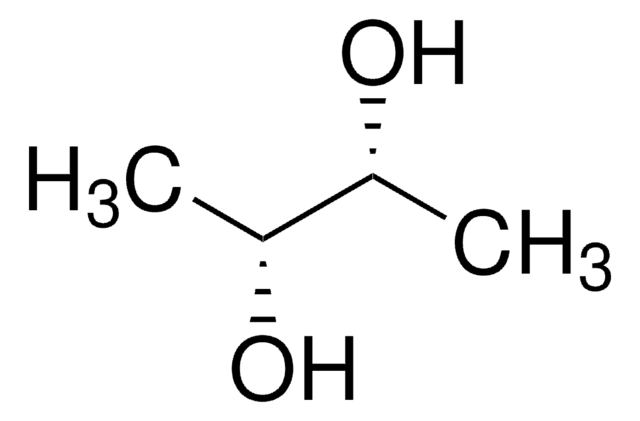
D-Threitol
99%
Synonym(s):
(2R,3R)-1,2,3,4-Butanetetrol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HOCH2[CH(OH)]2CH2OH
CAS Number:
Molecular Weight:
122.12
Beilstein:
1719752
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
optical activity
[α]20/D −14°, c = 2 in ethanol
mp
88-90 °C (lit.)
SMILES string
OC[C@@H](O)[C@H](O)CO
InChI
1S/C4H10O4/c5-1-3(7)4(8)2-6/h3-8H,1-2H2/t3-,4-/m1/s1
InChI key
UNXHWFMMPAWVPI-QWWZWVQMSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
N Tbeur et al.
Carbohydrate research, 329(2), 409-422 (2000-12-16)
A supported liquid membrane (SLM) containing a resorcinarene carrier has been used for the selective transport of erythritol, threitol, ribitol and xylitol from concentrated (1.0-0.01 M) aqueous solutions. The membrane is made of a microporous polytetrafluoroethylene film impregnated with a
F Bravo et al.
Carbohydrate research, 336(2), 83-97 (2001-11-02)
Differently protected erythro and threo furanoid glycals were synthesized by selenoxide elimination when phenyl 1-selenoglycosides were treated in oxidizing conditions (tBuOOH, Ti(O(i)Pr)(4), Et(2)(i)PrN). The phenyl 1-selenoglycosides were obtained from methyl 2-deoxy-D-erythro-pentofuranoside by protection of the primary hydroxyl or both hydroxyls
Justyna Wojno et al.
ACS chemical biology, 7(5), 847-855 (2012-02-14)
Invariant natural killer T (iNKT) cells are restricted by the non-polymorphic MHC class I-like protein, CD1d, and activated following presentation of lipid antigens bound to CD1d molecules. The prototypical iNKT cell agonist is α-galactosyl ceramide (α-GalCer). CD1d-mediated activation of iNKT
J M Borggreven et al.
Caries research, 26(2), 84-88 (1992-01-01)
Some newly designed surface-active phosphates were investigated with respect to their capacity to inhibit caries lesion formation in vitro. On the labial or buccal surfaces of sound human third molars, windows were prepared for investigation. One half of each window
Leonardo Silva Santos et al.
Rapid communications in mass spectrometry : RCM, 20(14), 2104-2108 (2006-06-13)
Recently, it has been proposed (M. Claeys et al., Science 2004; 303: 1173) that the atmospheric OH-radical-mediated photooxidation of isoprene is a source of two major secondary organic aerosol (SOA) components, that is, 2-methylthreitol and 2-methylerythritol. These diastereoisomeric tetrols, which
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service