All Photos(1)
About This Item
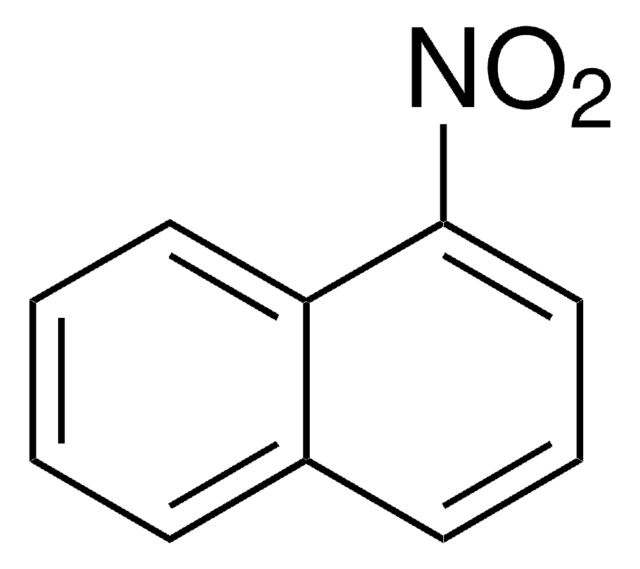
Linear Formula:
FC6H3(NO2)OH
CAS Number:
Molecular Weight:
157.10
Beilstein:
1870311
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
34-37 °C (lit.)
functional group
fluoro
nitro
SMILES string
Oc1cc(F)ccc1[N+]([O-])=O
InChI
1S/C6H4FNO3/c7-4-1-2-5(8(10)11)6(9)3-4/h1-3,9H
InChI key
QQURWFRNETXFTN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
5-Fluoro-2-nitrophenol has been used in:
- total synthesis of the (+/−)-CC-1065 CPI subunit
- synthesis of 2-(7-fluoro-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazin-6-yl)-4,5,6,7-tetrahydro-2H-isoindoline-1,3-diones
- 7-substituted 2H-1,4-benzoxazin-3-(4H)-ones, possessing therapeutic potential as inhibitors of angiogenesis
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
195.8 °F - closed cup
Flash Point(C)
91 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Michael D Ganton et al.
The Journal of organic chemistry, 72(2), 574-582 (2007-01-16)
CC-1065 and the related duocarmycins are members of a structurally unique family of naturally occurring molecules and remain some of the most rigorously studied antitumor compounds to date. Herein we describe a total synthesis of the (+/-)-CC-1065 CPI subunit in
Ming-Zhi Huang et al.
Journal of agricultural and food chemistry, 53(20), 7908-7914 (2005-09-30)
The mode of action of 2-(7-fluoro-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazin-6-yl)-4,5,6,7-tetrahydro-2H-isoindoline-1,3-diones, including the commercial herbicide flumioxazin, had been identified as inhibition of protoporphyrinogen oxidase (protox). As part of continuous efforts to search for new herbicides with high efficacy, broad-spectrum activity, and safety to crops, flumioxazin
Recent advances in the synthesis of 2H-1, 4-benzoxazin-3-(4H)-ones and 3, 4-dihydro-2 H -1, 4-benzoxazines.
Ilas J, et al.
Tetrahedron, 61(31), 7325-7348 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service