MSQC4
SILu™Lite SigmaMAb Universal Antibody Standard human
Synonym(s):
IgG1 light, Mass Spectrometry Universal Antibody Standard, SILu™Lite SigmaMAb Universal Antibody Standard human, recombinant IgG1 lambda light antibody, SigmaMAb
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
23201100
NACRES:
NA.12
Recommended Products
General description
SILU™ Lite SigmaMAb is a recombinant human monoclonal IgG1 lambda light antibody with a molecular mass of ∼150 kDa expressed in CHO cells. It is designed for optimization of accurate intact mass analysis of monoclonal antibodies, biosimilars, and pharmaceutical products. Accurate intact mass analysis of such large biomolecules can provide comprehensive information about structural and post-translational modifications such as glycosylation. Other information such as heterogeneity, batch-to-batch variation, amino acid truncation, and N-terminal Lys processing, aggregation, and degradation can be determined. Intact mass analysis using mass spectrometry is also very important for formulation and storage in therapeutic monoclonal antibody drug development.
It consists of two identical heavy chains and two identical light chains. The heavy chains and light chains are linked by one disulfide bond. The heavy chains are linked by two disulfide bonds located in a hinge domain. The other 12 cysteine bonds are intramolecularly restricted to six different globular domains. The antibody sequence has been evaluated by intact mass and peptide mapping using four different enzymes: chymotrypsin, Asp-N and Glu-C endoproteinases and trypsin. Sequence coverage of 100% was obtained.
It consists of two identical heavy chains and two identical light chains. The heavy chains and light chains are linked by one disulfide bond. The heavy chains are linked by two disulfide bonds located in a hinge domain. The other 12 cysteine bonds are intramolecularly restricted to six different globular domains. The antibody sequence has been evaluated by intact mass and peptide mapping using four different enzymes: chymotrypsin, Asp-N and Glu-C endoproteinases and trypsin. Sequence coverage of 100% was obtained.
Application
SILu™ Lite SigmaMAb Universal Antibody Standard human has been used as a model system to investigate the quantitative relationship between gas-phase monoclonal antibody (mAb) unfolding and the discrete levels of mAb glycosylation.
Features and Benefits
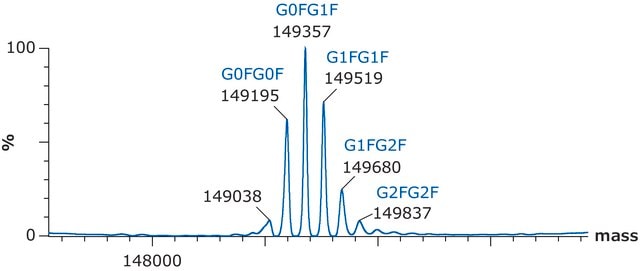
Calculated molecular weight values of the SigmaMAb light chains, heavy chains, and intact protein, with the most abundant glycoforms, are as follows:
Description / Composition / Modification / Average Mass (Da)
Light chain, reduced / C1006H1555N267O333S7 / Pyroglutamic acid (Q) / 22942.2
Heavy chain, reduced / C2181H3393N587O663S16 / (no modification) / 48957.8
C2237H3485N591O702S16 / G0F / 50403.2
C2243H3495N591O707S16 / G1F / 50565.3
C2249H3505N591O712S16 / G2F / 50727.5
Native intact mass, non-reduced / C6374H9864N1708O1992S46 / 2 X Pyroglutamic acid (Q) / 143767.7
C6486H10048N1716O2070S46 / G0F+G0F / 146658.4
C6492H10058N1716O2075S46 / G0F+G1F / 146820.6
C6498H10068N1716O2080S46 / G1F+G1F / 146982.7
C6504H10078N1716O2085S46 / G1F+G2F / 147144.8
C6510H10088N1716O2090S46 / G2F+G2F / 147307.0
Description / Composition / Modification / Average Mass (Da)
Light chain, reduced / C1006H1555N267O333S7 / Pyroglutamic acid (Q) / 22942.2
Heavy chain, reduced / C2181H3393N587O663S16 / (no modification) / 48957.8
C2237H3485N591O702S16 / G0F / 50403.2
C2243H3495N591O707S16 / G1F / 50565.3
C2249H3505N591O712S16 / G2F / 50727.5
Native intact mass, non-reduced / C6374H9864N1708O1992S46 / 2 X Pyroglutamic acid (Q) / 143767.7
C6486H10048N1716O2070S46 / G0F+G0F / 146658.4
C6492H10058N1716O2075S46 / G0F+G1F / 146820.6
C6498H10068N1716O2080S46 / G1F+G1F / 146982.7
C6504H10078N1716O2085S46 / G1F+G2F / 147144.8
C6510H10088N1716O2090S46 / G2F+G2F / 147307.0
Physical form
Supplied as a lyophilized powder containing phosphate buffered saline
Preparation Note
SigmaMAb recovery is maximized when phosphate buffer, pH 6–7, is used to reconstitute the lyophilized product.
Analysis Note
SigmaMab Heavy Chain
EVQLVESGGGLVQPGGSLRLSCVASGFTLNNYDMHWVRQGIGKGLEWVSKI
GTAGDRYYAGSVKGRFTISRENAKDSLYLQMNSLRVGDAAVYYCARGAGRW
APLGAFDIWGQGTMVTVSS|ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYF
PEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVN
HKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISR
TPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRV
VSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPS
RDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFL
YSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPG
SigmaMab Light Chain
QSALTQPRSVSGSPGQSVTISCTGTSSDIGGYNFVSWYQQHPGKAPKLMIY
DATKRPSGVPDRFSGSKSGNTASLTISGLQAEDEADYYCCSYAGDYTPGV
VFGGGTKLTVL|GQPKAAPSVTLFPPSSEELQANKATLVCLISDFYPGAVTV
AWKADSSPVKAGVETTTPSKQSNNKYAASSYLSLTPEQWKSHRSYSCQ
VTHEGSTVEKTVAPTECS
EVQLVESGGGLVQPGGSLRLSCVASGFTLNNYDMHWVRQGIGKGLEWVSKI
GTAGDRYYAGSVKGRFTISRENAKDSLYLQMNSLRVGDAAVYYCARGAGRW
APLGAFDIWGQGTMVTVSS|ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYF
PEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVN
HKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISR
TPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRV
VSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPS
RDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFL
YSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPG
SigmaMab Light Chain
QSALTQPRSVSGSPGQSVTISCTGTSSDIGGYNFVSWYQQHPGKAPKLMIY
DATKRPSGVPDRFSGSKSGNTASLTISGLQAEDEADYYCCSYAGDYTPGV
VFGGGTKLTVL|GQPKAAPSVTLFPPSSEELQANKATLVCLISDFYPGAVTV
AWKADSSPVKAGVETTTPSKQSNNKYAASSYLSLTPEQWKSHRSYSCQ
VTHEGSTVEKTVAPTECS
Also available as a stable isotope-labled product, Silu™Mab (Product Number MSQC3)
Package size based on protein content determined by A280
Other Notes
Avoid PBS for reconstitution.
Reconstitute the contents of the vial by adding 500μL of ultrapure water or phosphate buffer, and mixing vigorously. The solubilized product can be further diluted as needed.
Reconstitute the contents of the vial by adding 500μL of ultrapure water or phosphate buffer, and mixing vigorously. The solubilized product can be further diluted as needed.
Legal Information
SILu is a trademark of Sigma-Aldrich Co. LLC
related product
Product No.
Description
Pricing
supplement
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jared B Shaw et al.
Analytical chemistry, 90(18), 10819-10827 (2018-08-18)
Compared to traditional collision induced dissociation methods, electron capture dissociation (ECD) provides more comprehensive characterization of large peptides and proteins as well as preserves labile post-translational modifications. However, ECD experiments are generally restricted to the high magnetic fields of FTICR-MS
Daniel A Polasky et al.
Analytical chemistry, 91(4), 3147-3155 (2019-01-23)
Ion mobility-mass spectrometry (IM-MS) has become an important addition to the structural biology toolbox, but separating closely related protein conformations remain challenging. Collision-induced unfolding (CIU) has emerged as a valuable technique for distinguishing iso-cross-sectional protein and protein complex ions through
Yutong Jin et al.
mAbs, 11(1), 106-115 (2018-09-20)
The pharmaceutical industry's interest in monoclonal antibodies (mAbs) and their derivatives has spurred rapid growth in the commercial and clinical pipeline of these effective therapeutics. The complex micro-heterogeneity of mAbs requires in-depth structural characterization for critical quality attribute assessment and
"Collision Induced Unfolding Detects Subtle Differences in Intact Antibody Glycoforms and Associated Fragments.
Tian Y and Brandon T R
International Journal of Mass Spectrometry (2017)
John P McGee et al.
Journal of the American Society for Mass Spectrometry, 31(3), 763-767 (2020-03-05)
Intact protein mass spectrometry (MS) via electrospray-based methods is often degraded by low-mass contaminants, which can suppress the spectral quality of the analyte of interest via space-charge effects. Consequently, selective removal of contaminants by their mobilities would benefit native MS
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service