C3934
Carbonic Anhydrase from bovine erythrocytes
lyophilized powder, ≥2,000 W-A units/mg protein
Synonym(s):
Carbonate Dehydratase, Carbonate Hydrolyase
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Recommended Products
biological source
bovine erythrocytes
form
lyophilized powder
specific activity
≥2,000 W-A units/mg protein
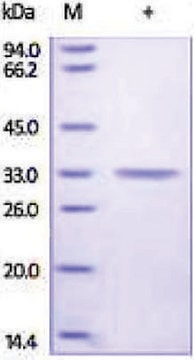
mol wt
30 kDa
application(s)
diagnostic assay manufacturing
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
Application
Carbonic anhydrase from bovine erythrocytes (BCA) has been used to study the effect of removal of enzyme-bound metal ion, Zn2+, on aggregation behavior of the enzyme. Removal of metal ion by a chelator such as EDTA enhances the propensity of the enzyme to adopt the molten-globule state. This state of the enzyme is found to bind to the chaperone-like α-crystallin and prevent aggregation. The enzyme from Sigma has been immobilized to electrochemical transducers in order to obtain develop an analytical device for dissolved CO2 measurement. It has also been used for the thermodynamic analysis of conformational changes in BCA.
Biochem/physiol Actions
Carbonic anhydrase is a zinc metalloenzyme that has a molecular weight of approximately 30,000 Da. The enzyme catalyzes the hydration of carbon dioxide to carbonic acid. It is involved in vital physiological and pathological processes such as pH and CO2 homeostasis, transport of bicarbonate and CO2, biosynthetic reactions, bone resorption, calcification, and tumorigenicity. Therefore, this enzyme is an important target for inhibitors with clinical applications for various pathologies such as glaucoma, epilepsy and Parkinson′s disease.
Unit Definition
One Wilbur-Anderson (W-A) unit will cause the pH of a 0.02 M Trizma buffer to drop from 8.3 to 6.3 per min at 0°C. (One W-A unit is essentially equivalent to one Roughton-Booth unit.)
inhibitor
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fraser J Moss et al.
The Journal of physiology, 598(24), 5821-5856 (2020-09-25)
According to the HCO 3 - metabolon hypothesis, direct association of cytosolic carbonic anhydrases (CAs) with the electrogenic Na/HCO3 cotransporter NBCe1-A speeds transport by regenerating/consuming HCO 3 - . The present work addresses published discrepancies as to whether
Use of carbonic anhydrase in electrochemical biosensors for dissolved CO2
Cammaroto C, et al.
Sensors and Actuators B, Chemical, 48(1-3), 439-447 (1998)
Mikhail Krasavin et al.
Journal of enzyme inhibition and medicinal chemistry, 35(1), 165-171 (2019-11-23)
Testing of an expanded, 800-compound set of analogues of the earlier described Strecker-type α-aminonitriles (selected from publicly available Enamine Ltd. Screening Collection) in thermal shift assay against bovine carbonic anhydrase (bCA) led to further validation of this new class of
Angelina M Dichiera et al.
The Journal of experimental biology, 223(Pt 22) (2020-11-28)
Oxygen (O2) and carbon dioxide (CO2) transport are tightly coupled in many fishes as a result of the presence of Root effect hemoglobins (Hb), whereby reduced pH reduces O2 binding even at high O2 tensions. Red blood cell carbonic anhydrase
Heather N Hollowell et al.
Journal of biochemistry and molecular biology, 40(2), 205-211 (2007-03-31)
The stability curve - a plot of the Gibbs free energy of unfolding versus temperature - is calculated for bovine erythrocyte carbonic anhydrase in 150 mM sodium phosphate (pH = 7.0) from a combination of reversible differential scanning calorimetry measurements
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service