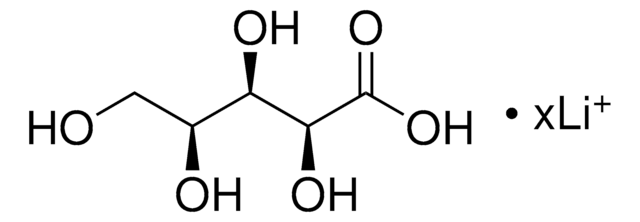
73671
D-Xylonic acid lithium salt
≥95.0% (TLC)
Synonym(s):
Lithium D-xylonate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
Quality Level
Assay
≥95.0% (TLC)
form
powder or crystals
optical activity
[α]/D 18±2° in H2O
color
white to off-white
storage temp.
2-8°C
SMILES string
OC[C@@H](O)[C@H](O)[C@@H](O)C(O)=O
InChI
1S/C5H10O6/c6-1-2(7)3(8)4(9)5(10)11/h2-4,6-9H,1H2,(H,10,11)/t2-,3+,4-/m1/s1
InChI key
QXKAIJAYHKCRRA-FLRLBIABSA-N
Biochem/physiol Actions
Metabolite of pentose and glucuronate interconversions
Other Notes
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yvonne Nygård et al.
Metabolic engineering, 25, 238-247 (2014-07-30)
D-xylonate is a potential platform chemical which can be produced by engineered Saccharomyces cerevisiae strains. In order to address production constraints in more detail, we analysed the role of lactone ring opening in single cells and populations. Both D-xylono-γ-lactone and
Mervi Toivari et al.
Bioresource technology, 133, 555-562 (2013-03-05)
D-xylonic acid is one of the top 30 most desirable chemicals to be derived from biomass sugars identified by the US Department of Energy, being applicable as a non-food substitute for D-gluconic acid and as a platform chemical. We engineered
Wei Niu et al.
Journal of the American Chemical Society, 125(43), 12998-12999 (2003-10-23)
The lack of a route to precursor 1,2,4-butanetriol that is amenable to large-scale synthesis has impeded substitution of 1,2,4-butanetriol trinitrate for nitroglycerin. To identify an alternative to the current commercial synthesis of racemic d,l-1,2,4-butanetriol involving NaBH4 reduction of esterified d,l-malic
Note on D-xylonate utilization by ascomycetous and basidiomycetous yeasts.
Van Der Walt, J. P. and Steyn, R. L.
Systematic and Applied Microbiology, 13, 192-193 (1990)
Huaiwei Liu et al.
Bioresource technology, 115, 244-248 (2011-09-16)
An engineered Escherichia coli was constructed to produce D-xylonic acid, one of the top 30 high-value chemicals identified by US Department of Energy. The native pathway for D-xylose catabolism in E. coli W3110 was blocked by disrupting xylose isomerase (XI)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service