D133809
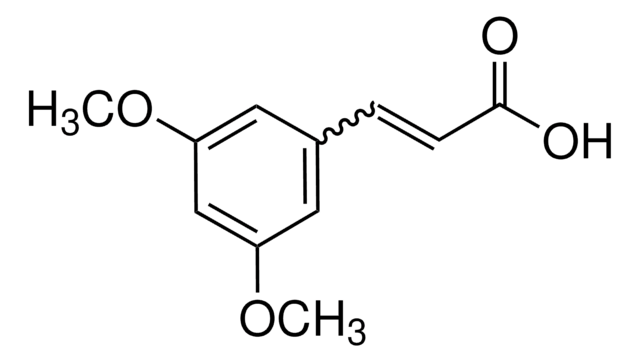
3,4-Dimethoxycinnamic acid, predominantly trans
99%
Synonym(s):
Caffeic acid dimethyl ether
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3O)2C6H3CH=CHCO2H
CAS Number:
Molecular Weight:
208.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
powder
mp
181-183 °C (lit.)
SMILES string
COc1ccc(\C=C\C(O)=O)cc1OC
InChI
1S/C11H12O4/c1-14-9-5-3-8(4-6-11(12)13)7-10(9)15-2/h3-7H,1-2H3,(H,12,13)/b6-4+
InChI key
HJBWJAPEBGSQPR-GQCTYLIASA-N
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Thiago Nogueira et al.
Electrophoresis, 28(19), 3570-3574 (2007-09-05)
A method based on the formation of pi-complexes with chlorogenate-like species was proposed for the determination of caffeine in regular (nondecaffeinated) and decaffeinated coffee. Both caffeate and 3,4-dimethoxycinnamate were able to transform caffeine--a neutral species in aqueous solutions--into an anionic
[Chemical constituents of Veronicastrum sibiricum (L.) Pennell].
B Zhou et al.
Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica, 17(1), 35-36 (1992-01-01)
Tracy L Farrell et al.
Molecular nutrition & food research, 56(9), 1413-1423 (2012-08-07)
This study reports the 24 h human plasma pharmacokinetics of 3,4-dimethoxycinnamic acid (dimethoxycinnamic acid) after consumption of coffee, and the membrane transport characteristics of certain dimethoxycinnamic acid derivatives, as present in coffee. Eight healthy human volunteers consumed a low-polyphenol diet
B Zhou et al.
Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica, 17(8), 493-496 (1992-08-01)
Six compounds were isolated from Veronicastrum sibiricum and identified as D-mannitol, beta-sitosterol, daucosterine, 3-0-acetyloleanolic acid, 3, 4-dimethoxy cinnamic acid and isoferulic acid. Pharmacological experiment has shown that isoferulic acid is anti-inflammatory and analgesic; 3-0-acetyloleanolic acid is anti-inflammatory; mannitol is analgesic
Maher M El-Domiaty et al.
Zeitschrift fur Naturforschung. C, Journal of biosciences, 64(1-2), 11-19 (2009-03-28)
A new natural compound, named 6-O-(3",4"-dimethoxycinnamoyl) catalpol, was isolated from the defatted alcoholic extract of the flowering parts of Buddleja asiatica Lour. (family Scrophulariaceae). Other separated known compounds included steroids (beta-sitosterol, stigmasterol, stigmasterol-O-glucoside, beta-sitosterol-O-glucoside), iridoid glucosides (methyl catalpol, catalpol, aucubin)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service