89761
N-Boc- 2,2′-(ethylenedioxy)diethylamine
≥95.0% (NT), for peptide synthesis
Synonym(s):
N-Boc-3,6-dioxa-1,8-octanediamine, tert-Butyl 2-[2-(2-aminoethoxy)ethoxy]ethylcarbamate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
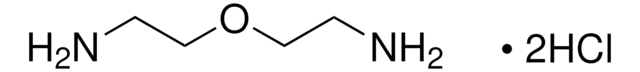
C11H24N2O4
CAS Number:
Molecular Weight:
248.32
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Product Name
N-Boc- 2,2′-(ethylenedioxy)diethylamine, ≥95.0% (NT)
Assay
≥95.0% (NT)
form
liquid
reaction suitability
reagent type: cross-linking reagent
density
1.046 g/mL at 20 °C
functional group
Boc
amine
SMILES string
NCCOCCOCCNC(OC(C)(C)C)=O
InChI
1S/C11H24N2O4/c1-11(2,3)17-10(14)13-5-7-16-9-8-15-6-4-12/h4-9,12H2,1-3H3,(H,13,14)
InChI key
OCUICOFGFQENAS-UHFFFAOYSA-N
Related Categories
Application
Linker reagent for the prep. of a broad variety of linked tags, monomers for polymerisation, ionophores etc., e.g. biotinylation reagents.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Braun, M., et al.
European Journal of Organic Chemistry, 7, 1173 - 1182 (2000)
Sigal, G. B., et al.
Journal of the American Chemical Society, 118, 3789-3800 (1996)
Michelle Trester-Zedlitz et al.
Journal of the American Chemical Society, 125(9), 2416-2425 (2003-02-27)
A method is described for the elucidation of protein-protein interactions using novel cross-linking reagents and mass spectrometry. The method incorporates (1) a modular solid-phase synthetic strategy for generating the cross-linking reagents, (2) enrichment and digestion of cross-linked proteins using microconcentrators
Spinke, J., et al.
The Journal of Physical Chemistry, 99(9), 7012-7019 (1993)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service