634492
4-Pyridinylboronic acid
90%
Synonym(s):
4-Pyridineboronic acid, 4-Pyridylboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C5H6BNO2
CAS Number:
Molecular Weight:
122.92
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
90%
form
solid
mp
>300 °C (lit.)
storage temp.
−20°C
SMILES string
OB(O)c1ccncc1
InChI
1S/C5H6BNO2/c8-6(9)5-1-3-7-4-2-5/h1-4,8-9H
InChI key
QLULGIRFKAWHOJ-UHFFFAOYSA-N
Related Categories
General description
4-Pyridinylboronic acid is commonly used as a reagent in cross-coupling reactions such as Suzuki-Miyaura cross-coupling.
Application
Reagent used for
Reagent used in Preparation of
- Palladium-catalyzed Suzuki-Miyaura coupling reactions
- Ligand-free palladium-catalyzed Suzuki coupling reaction under microwave irradation
Reagent used in Preparation of
- HIV-1 protease inhibitors
- Potential cancer threapeutics, such as PDK1 and protein kinase CK2 inhibitors
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
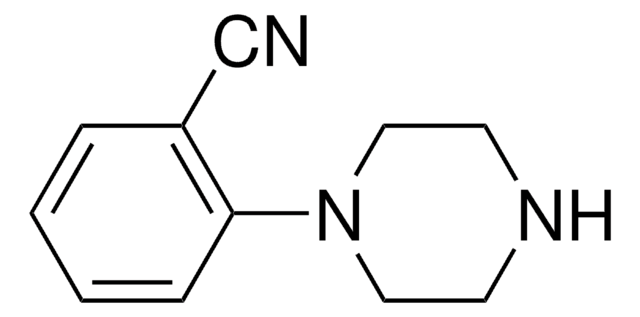
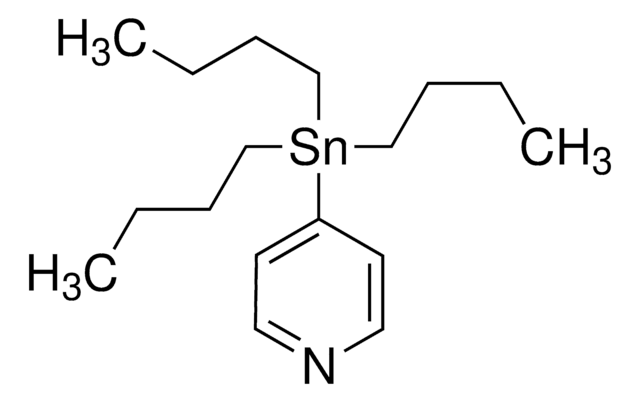
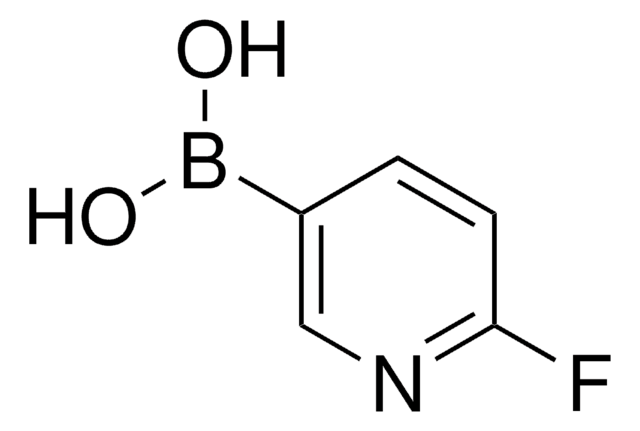
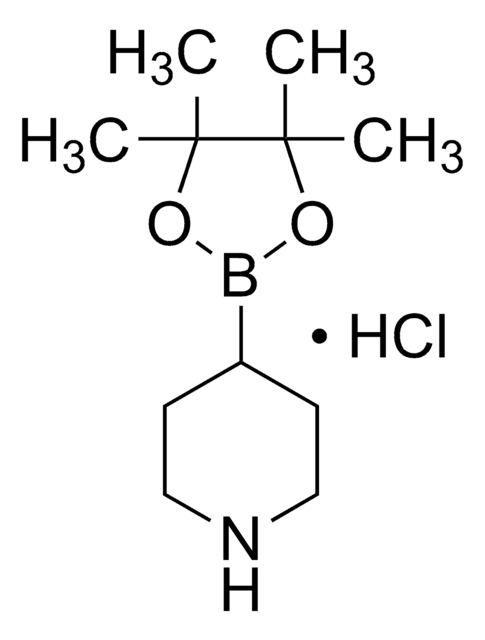
Customers Also Viewed
Jin-Tao Yu et al.
Organic & biomolecular chemistry, 10(7), 1359-1364 (2011-12-20)
An efficient palladium-catalyzed Suzuki-Miyaura coupling method involving the reaction between CTV-Br(3) and a variety of aryl and heteroaryl boronic acids in the presence of indolyl phosphane ligands has been developed. This reaction procedure provided a series of C(3)-symmetric aryl-extended rigid
Sumin Lee et al.
Organic letters, 14(9), 2238-2241 (2012-04-28)
The kinetic process of key intermediates involved in the electrochemical ring opening of photochromic dithienylcyclopentenes (DTEs) has been observed for the first time, where the electronic nature of the DTEs is an important factor that determines the rate-determining step in
Jorge Cruz-Huerta et al.
Chemical communications (Cambridge, England), 48(35), 4241-4243 (2012-03-23)
The combination of two heteroaromatic boronic acids with pentaerythritol gave self-complementary tectons which were suitable for the generation of 2D and 3D molecular networks.
One-pot approach to N-quinolyl 3?/4?-biaryl carboxamides by microwave-assisted Suzuki--Miyaura coupling and N-boc deprotection
ZY Huang, et al.
The Journal of Organic Chemistry, 81, 9647-9657 (2016)
Sarah J Vella et al.
Beilstein journal of organic chemistry, 14, 1908-1916 (2018-08-17)
A two-station [2]catenane containing a large macrocycle with two different recognition sites, one bis(pyridinium)ethane and one benzylanilinium, as well as a smaller DB24C8 ring was synthesized and characterized. 1H NMR spectroscopy showed that the DB24C8 ring can shuttle between the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service