All Photos(1)
About This Item
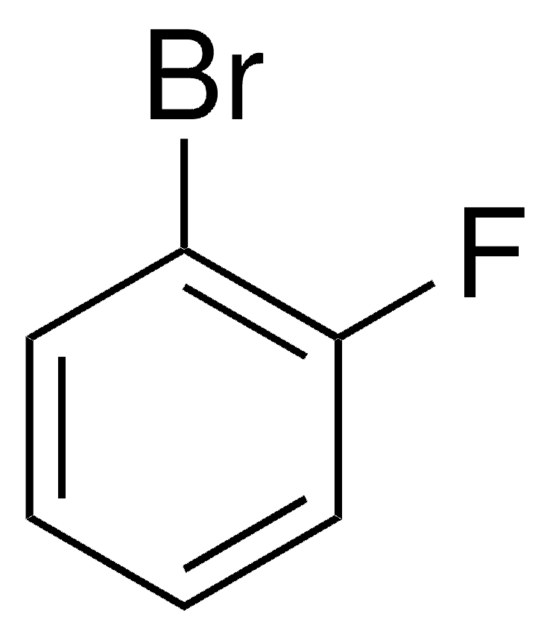
Linear Formula:
BrC6H3(F)NO2
CAS Number:
Molecular Weight:
220.00
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
refractive index
n20/D 1.575 (lit.)
bp
240-241 °C (lit.)
mp
18-19 °C (lit.)
density
1.786 g/mL at 25 °C (lit.)
SMILES string
[O-][N+](=O)c1cc(Br)ccc1F
InChI
1S/C6H3BrFNO2/c7-4-1-2-5(8)6(3-4)9(10)11/h1-3H
InChI key
UQEANKGXXSENNF-UHFFFAOYSA-N
General description
4-Bromo-1-fluoro-2-nitrobenzene undergoes Sonogashira reaction with 2-fluoronitrobenzene to afford predominantly the bromo displacement product.
Application
4-Bromo-1-fluoro-2-nitrobenzene may be used in the synthesis of:
- 6-bromo-1H-benzo[d][1,2,3]triazol-1-ol
- 2-(4-bromo-2-nitrophenylamino)-5-methylthiophene-3-carbonitrile
- dibenzoxazepine analog, as potent sodium channel blocker
- 4-(4-bromo-2-nitrophenyl)piperazine-1-carboxylic acid tert-butylester
Used in the synthesis of anti-inflammatory agents.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Patrick L DeRoy et al.
Organic letters, 9(14), 2741-2743 (2007-06-08)
The nucleophilic aromatic substitution reaction between electron-deficient aryl fluorides and terminal alkynes is shown to be efficiently promoted by sodium bis(trimethylsilyl)amide as a base. Moderate to excellent yields of 2-ethynylnitrobenzene products can be obtained under mild conditions.
Katie M Lutker et al.
Crystal growth & design, 8(1), 136-139 (2008-01-01)
Bis(5-methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrilyl)acetylene, a derivative of the highly polymorphic compound 5-methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile (ROY) that possesses two chromophores electronically coupled through a triple bond, was found to be trimorphic. Structural data for two of these forms indicates that symmetry is maintained in one structure
Venkateshwarlu Gurram et al.
Advanced synthesis & catalysis, 357(2-3), 451-462 (2015-03-03)
Benzotriazoles are a highly important class of compounds with broad-ranging applications in such diverse areas as medicinal chemistry, as auxiliaries in organic synthesis, in metallurgical applications, in aircraft deicing and brake fluids, and as antifog agents in photography. Although there
Tomoki Kawai et al.
Nuclear medicine and biology, 40(5), 705-709 (2013-05-28)
As a first trial for in vivo imaging of β-secretase (BACE1) in Alzheimer's disease brain, we applied a novel non-peptidergic small molecule which has high affinity to the enzyme, naphthalene-1-carboxylic acid (3'-chloro-4'-fluoro-4-piperazin-1-yl-biphenyl-3-yl)amide (NCFB) into positron emission tomography (PET) probe. In
Stephen M Lynch et al.
Bioorganic & medicinal chemistry letters, 25(1), 43-47 (2014-12-04)
We have identified two related series of dibenzazepine and dibenzoxazepine sodium channel blockers, which showed good potency on Nav1.7 in FLIPR-based and electrophysiological functional assays.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)