All Photos(1)
About This Item
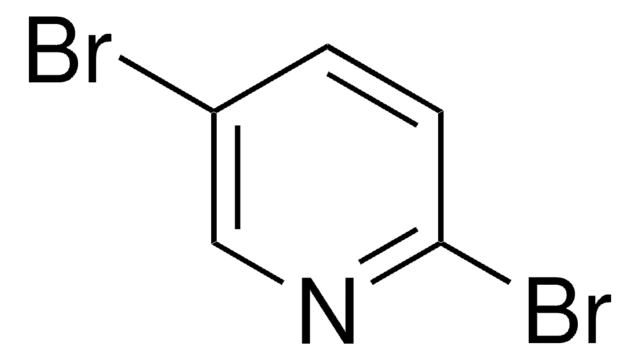
Empirical Formula (Hill Notation):
C5H5IN2
CAS Number:
Molecular Weight:
220.01
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
128-131 °C (lit.)
SMILES string
Nc1ccc(I)cn1
InChI
1S/C5H5IN2/c6-4-1-2-5(7)8-3-4/h1-3H,(H2,7,8)
InChI key
IVILGUFRMDBUEQ-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R J Bochis et al.
Journal of medicinal chemistry, 21(2), 235-237 (1978-02-01)
A series of methyl imidazo-[11,2-a]pyridine-2-carbamates was synthesized for anthelmintic testing. The preparation of this class of compounds was simplified by utilization of a novel one-step condensation of the appropriately substituted 2-aminopyridine and methyl chloroacetylcarbamate. The most potent compound, methyl 6-(phenylsulfinyl)-imidazo[1,2-a]pyridine-2-carbamate
N Sundaraganesan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 67(3-4), 830-836 (2006-10-05)
The Fourier transform Raman and Fourier transform infrared spectra of 2-amino-5-iodopyridine were recorded in the solid phase. The equilibrium geometry, harmonic vibrational frequencies, infrared intensities and Raman scattering activities were calculated by HF and DFT (B3LYP) methods with the 6-31G(d,p)
Transition metal halide salts of 2-amino-5-substituted-pyridines: Synthesis, crystal structure and magnetic properties of two polymorphs of (5-IAP)2CuCl4 [5-IAP= 2-amino-5-iodopyridinium].
Giantsidis J, et al.
Journal of Coordination Chemistry, 55(7), 795-803 (2002)
Zhi-Ping Zhuang et al.
Journal of medicinal chemistry, 46(2), 237-243 (2003-01-10)
A series of novel beta-amyloid (A beta) aggregate-specific ligands, 2-(4'-dimethylaminophenyl)-6-iodoimidazo[1,2-a]pyridine, 16(IMPY), and its related derivatives were prepared. An in vitro binding study with preformed A beta aggregates showed that 16(IMPY) and its bromo derivative competed with binding of 2-(4'-dimethylaminophenyl)-6-iodobenzothiazole, [125I]7(TZDM)
Substituted 2-Sulfonamido-5-aminopyridines. II.
Caldwell WT, et al.
Journal of the American Chemical Society, 66(9), 1479-1484 (1944)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service