All Photos(2)
About This Item
Empirical Formula (Hill Notation):
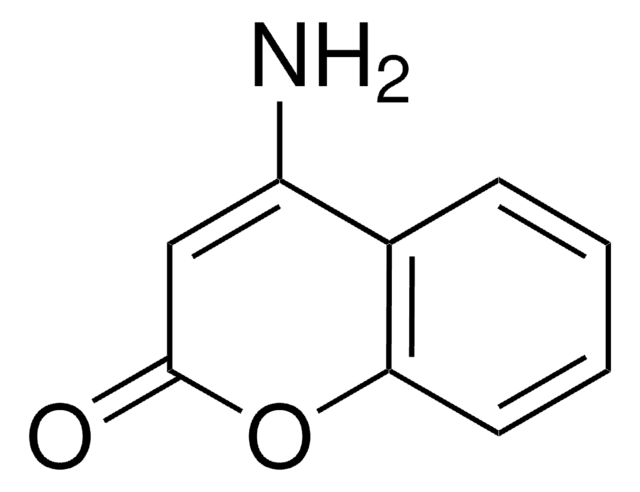
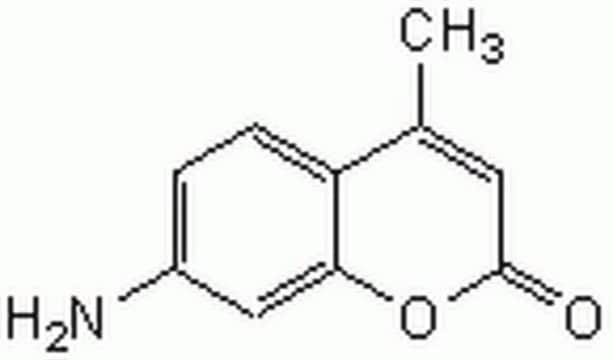
C9H7NO2
CAS Number:
Molecular Weight:
161.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
135-139 °C (lit.)
SMILES string
NC1=Cc2ccccc2OC1=O
InChI
1S/C9H7NO2/c10-7-5-6-3-1-2-4-8(6)12-9(7)11/h1-5H,10H2
InChI key
QWZHDKGQKYEBKK-UHFFFAOYSA-N
Application
3-Aminocoumarin may be employed in the following studies:
- As ligand for the synthesis of Cr(III), Ni(II), and Cu(II) complexes.
- As starting reagent for the synthesis of 1,2,3,4-tetrahydropyrido[2,3-c]coumarins.
- Synthesis of new 3-(heteroaryl)aminocoumarin derivatives, via optimized Buchwald-Hartwig amination reaction.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Oscar Aguilar-Sopeña et al.
International journal of molecular sciences, 21(7) (2020-04-09)
We have previously shown the delivery of phosphatase of regenerating liver-1 (PRL-1) to the immunological synapse (IS) and proposed a regulatory role of the catalytic activity of PRLs (PRL-1, PRL-2 and PRL-3) in antigen-induced IL-2 production. Nonetheless, the expression in
Iu A Vladimirov et al.
Biulleten' eksperimental'noi biologii i meditsiny, 112(10), 358-360 (1991-10-01)
The antioxidant capacity of 3-aminocoumarin, 3-oxycoumarin, 3-acetylaminocoumarin, and 3-coumarin carbonic acid has been investigated with chemiluminescence measurement and by the accumulation of TBA-active products. All coumarins were found to be antioxidants, with 3-oxy-, 3-amino- and 3-acetylamino coumarins being capable of
Abdul Amir H Kadhum et al.
Molecules (Basel, Switzerland), 16(8), 6969-6984 (2011-08-17)
3-Aminocoumarin (L) has been synthesized and used as a ligand for the formation of Cr(III), Ni(II), and Cu(II) complexes. The chemical structures were characterized using different spectroscopic methods. The elemental analyses revealed that the complexes where M=Ni(II) and Cu(II) have
Synthesis of biologically potent new 3-(heteroaryl) aminocoumarin derivatives via Buchwald-Hartwig C-N coupling.
Das AR, et al.
Tetrahedron Letters, 51(7), 1099-1102 (2010)
Wei Jia et al.
Analytica chimica acta, 957, 29-39 (2017-01-22)
An analytical method was developed and validated for simultaneous analysis of one hundred and thirty-seven veterinary drug residues and metabolites from sixteen different classes in tilapia utilizing an improved fully non-targeted way of data acquisition with fragmentation. The automated on-line
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service