158518
Phenoxyacetic acid
98%
Synonym(s):
Glycolic acid phenyl ether
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
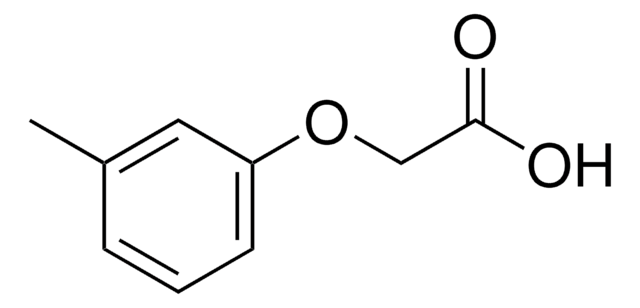
C6H5OCH2CO2H
CAS Number:
Molecular Weight:
152.15
Beilstein:
907949
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
98-100 °C (lit.)
solubility
ethanol: soluble 10%, clear, colorless
SMILES string
OC(=O)COc1ccccc1
InChI
1S/C8H8O3/c9-8(10)6-11-7-4-2-1-3-5-7/h1-5H,6H2,(H,9,10)
InChI key
LCPDWSOZIOUXRV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Phenoxyacetic acid is a herbicide and its photodegradation using TiO2 as photocatalyst has been studied. Selective separation of penicillin V from phenoxyacetic acid using liquid membranes consisting of 1,2-dichloroethane and Amberlite LA-2 as carrier has been studied.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Rudina Bleta et al.
Langmuir : the ACS journal of surfaces and colloids, 30(39), 11812-11822 (2014-09-16)
A series of mesoporous titania photocatalysts with tailorable structural and textural characteristics was prepared in aqueous phase via a colloidal self-assembly approach using various cyclodextrins (CDs) as structure-directing agents. The photocatalysts and the structure-directing agents were characterized at different stages
Photocatalyzed Degradation of Phenol, 2, 4-Dichlorophenol, Phenoxyacetic Acid and 2, 4-Dichlorophenoxyacetic Acid over SupportedTiO2 in a Flow System.
Trillas M, et al.
Journal of Chemical Technology and Biotechnology, 67(3), 237-242 (1996)
Selective separation of penicillin V from phenoxyacetic acid using liquid membranes.
Cascaval D, et al.
Biochemical Engineering Journal, 55(1), 45-50 (2000)
Charles Timchalk
Toxicology, 200(1), 1-19 (2004-05-26)
Phenoxyacetic acids including 2,4-dichlorophenoxyacetic acid (2,4-D) and 4-chloro-2-methylphenoxyacetic acid (MCPA) are widely utilized organic acid herbicides that have undergone extensive toxicity and pharmacokinetic analyses. The dog is particularly susceptible to the toxicity of phenoxyacetic acids and related organic acids relative
Mohamed Ashraf Ali et al.
Bioorganic & medicinal chemistry, 15(5), 1896-1902 (2007-01-26)
A series of 2-{4-[1-amino (thioxo) methyl-5-(substituted phenyl)-4,5-dihydro-1H-3-pyrazolyl]-2-methoxyphenoxy}acetic acid and 2-{4-[1-carbamoyl-5-(substituted phenyl)-4,5-dihydro-1H-3-pyrazolyl]-2-methoxyphenoxy}acetic acid were synthesized and the in vitro activity of the synthesized compounds against Mycobacterium tuberculosis H37Rv (MTB) and INH-resistant M. tuberculosis (INHR-MTB) was studied. Among the synthesized compounds, compound
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)