All Photos(1)
About This Item
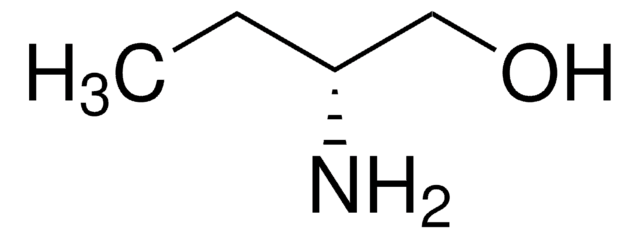
Linear Formula:
C2H5CH(NH2)CH2OH
CAS Number:
Molecular Weight:
89.14
Beilstein:
1718930
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98%
form
liquid
optical activity
[α]20/D +10°, neat
optical purity
ee: 96% (GLC)
refractive index
n20/D 1.4521 (lit.)
bp
172-174 °C (lit.)
density
0.944 g/mL at 25 °C (lit.)
functional group
amine
hydroxyl
SMILES string
CC[C@H](N)CO
InChI
1S/C4H11NO/c1-2-4(5)3-6/h4,6H,2-3,5H2,1H3/t4-/m0/s1
InChI key
JCBPETKZIGVZRE-BYPYZUCNSA-N
Looking for similar products? Visit Product Comparison Guide
Application
(S)-(+)-2-Amino-1-butanol is a chiral amino alcohol that may be used in the synthesis of (S)-2-(6-benzylamino-9-isopropyl-9H-purin-2-ylamino) butan-1-ol, an (S)-enantiomer of roscovitine. It can also be used to synthesize homochiral 2-methylpyrroles. (S)-(+)-2-Amino-1-butanol is an intermediate for the synthesis of (S,S)-ethambutol, an antibacterial agent for treating tuberculosis.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
203.0 °F - closed cup
Flash Point(C)
95 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and configuration of the cyclin-dependent kinase inhibitor roscovitine and its enantiomer.
Wang S, et al
Tetrahedron Asymmetry, 12(20), 2891-2894 (2001)
Synthesis and photooxygenation of homochiral 2-methylpyrrole derivatives of chiral amino alcohols: simple, selective access to chiral bicyclic lactams
Aydogan F and Demir AS
Tetrahedron Asymmetry, 15(2), 259-265 (2004)
Enantioselective synthesis of (S, S)-ethambutol using proline-catalyzed asymmetric a-aminooxylation and a-amination
Kotkar SP and Sudalai A
Tetrahedron Asymmetry, 17(11), 1738-1742 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service