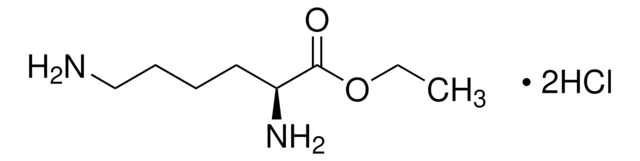
L0645
L-Lysine methyl ester dihydrochloride
≥99% (TLC)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H16N2O2 · 2HCl
CAS Number:
Molecular Weight:
233.14
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
product name
L-Lysine methyl ester dihydrochloride,
Assay
≥99% (TLC)
Quality Level
form
powder
color
white
storage temp.
−20°C
SMILES string
Cl.COC(=O)C(N)CCCCN
InChI
1S/C7H16N2O2.ClH/c1-11-7(10)6(9)4-2-3-5-8;/h6H,2-5,8-9H2,1H3;1H
InChI key
FORVAIDSGSLRPX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
L-Lysine methyl ester is used in the production of lysine methyl ester based cationic surfactants and hydrogels.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C O Mills et al.
Biochimica et biophysica acta, 1336(3), 485-496 (1997-11-21)
We have synthesised and characterised a fluorescent monohydroxy bile salt analogue, lithocholyl-lysyl-fluorescein and compared its physical and biological properties with those of lithocholate, glycolithocholate, sulpholithocholate, lithocholic acid glucuronide and taurocholate. The synthetic method used excess N-epsilon-CBZ-L-lysine methyl ester hydrochloride and
Atoosa Maleki et al.
Carbohydrate research, 342(18), 2776-2792 (2007-09-29)
Dynamic light scattering (DLS) and rheological experiments have been performed on semidilute aqueous hyaluronic acid (HA) solutions during the chemical cross-linking process with a water-soluble carbodiimide (WSC) to produce a hydrogel. The formation and destruction of the gel are characterized.
Aurora Pinazo et al.
Langmuir : the ACS journal of surfaces and colloids, 25(14), 7803-7814 (2009-07-15)
The synthesis of a novel class of lysine-based cationic amphiphilic derivatives of the type N(epsilon),N(epsilon)'-bis(n-acyloxypropyl)-l-lysine methyl ester salts combining several hydroxyl functions and aliphatic chains of 12 or 14 carbon atoms is described. The compounds have one, two, three, and
Yujia Zhang et al.
Journal of materials chemistry. B, 8(33), 7428-7437 (2020-07-15)
Infectious diseases induced by pathogenic bacteria are the major causes for the failure of medical implants. Meanwhile, the drug-resistance is steadily developed because of the large and even inappropriate use of antibiotics. Therefore, the development of antibacterial coating with non-antibiotic-based
Dávid Juriga et al.
Beilstein journal of nanotechnology, 10, 2579-2593 (2020-01-11)
Polymer hydrogels are ideal scaffolds for both tissue engineering and drug delivery. A great advantage of poly(amino acid)-based hydrogels is their high similarity to natural proteins. However, their expensive and complicated synthesis often limits their application. The use of poly(aspartic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service