220201
CHAPS
Molecular Biology Grade
Synonym(s):
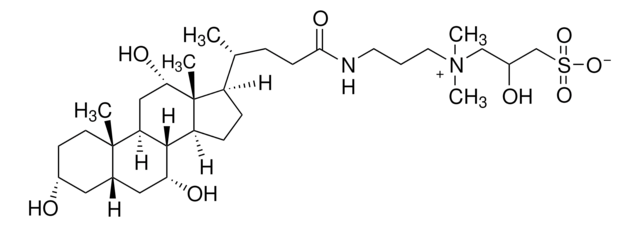
CHAPS, 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate
About This Item
Recommended Products
product name
CHAPS, Molecular Biology Grade,
grade
Molecular Biology
Quality Level
description
zwitterionic
Assay
≥98% (NMR)
form
crystalline powder
mol wt
micellar avg mol wt 6150
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
desiccated (hygroscopic)
aggregation number
10
color
white
CMC
6 - 10 mM
6 mM (20-25°C)
mp
157 °C (315 °F)
transition temp
cloud point >100 °C
solubility
water: 1.0 M
foreign activity
DNase, RNase, and Protease, none detected
shipped in
ambient
storage temp.
15-25°C
SMILES string
C[C@H](CCC(=O)NCCC[N+](C)(C)CCCS([O-])(=O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)CC4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C
InChI
1S/C32H58N2O7S/c1-21(8-11-29(38)33-14-6-15-34(4,5)16-7-17-42(39,40)41)24-9-10-25-30-26(20-28(37)32(24,25)3)31(2)13-12-23(35)18-22(31)19-27(30)36/h21-28,30,35-37H,6-20H2,1-5H3,(H-,33,38,39,40,41)/t21-,22?,23-,24-,25+,26+,27-,28+,30+,31+,32-/m1/s1
InChI key
UMCMPZBLKLEWAF-RFCNGIAKSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Useful for solubilizing membrane proteins and breaking protein-protein interactions. CHAPS′ small micellar molecular weight (6,150) and high critical micelle concentration (6-10 mM) allow it to be removed from samples by dialysis. It is also suitable for protein solubilization for isoelectric focusing and two-dimensional electrophoresis. CHAPS is commonly used for non-denaturing (without urea) IEF and has been shown to give excellent resolution of some subcellular preparations and plant proteins. Concentrations between 2-4% (w/v) are typically used in an IEF gel.
Warning
Reconstitution
Other Notes
Ofri, D., et al. 1992. J. Neurochem. 58, 628.
Ransom, R.W., et al. 1992. Biochem. Pharmacol. 43, 1823.
Simonds, W.F., et al. 1992. Proc. Natl. Acad. Sci. USA77, 4623.
Yannariello-Brown, J., and Weigel, P.H. 1992. Biochemistry31, 576.
Hjelmeland, L.M. 1980. Proc. Natl. Acad. Sci. USA77, 6368.
Simonds, W.F., et al. 1980. Proc. Natl. Acad. Sci. USA77, 4623.
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![CHAPS (3-[(3-Cholamidopropyl)-dimethylammonio]-propane- sulfonate) for biochemistry](/deepweb/assets/sigmaaldrich/product/structures/265/550/452ffcaa-af89-4e31-99a1-0a0f29072b82/640/452ffcaa-af89-4e31-99a1-0a0f29072b82.png)