P33429
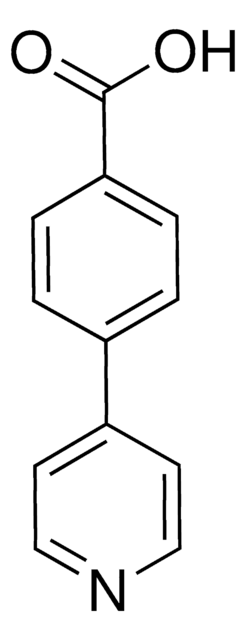
4-Phenylpyridine
97%
Synonym(s):
γ-Phenylpyridine, 1-Benzylhydrazine dihydrochloride, p-Phenylpyridine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C11H9N
CAS Number:
Molecular Weight:
155.20
Beilstein:
110490
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
crystals
bp
274-275 °C (lit.)
mp
69-73 °C (lit.)
SMILES string
c1ccc(cc1)-c2ccncc2
InChI
1S/C11H9N/c1-2-4-10(5-3-1)11-6-8-12-9-7-11/h1-9H
InChI key
JVZRCNQLWOELDU-UHFFFAOYSA-N
Gene Information
human ... MMP3(4314)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
K S Hsu et al.
The Journal of pharmacology and experimental therapeutics, 279(2), 740-747 (1996-11-01)
The effect of L-deprenyl (selegiline) on the excitatory synaptic transmission was characterized in the CA1 neurons of rat hippocampal slices by using a intracellular recording technique. Superfusion of L-deprenyl (0.1-10 microM) reversibly decreased the EPSP, which was evoked by orthodromic
Germano Giuliani et al.
Bioorganic & medicinal chemistry, 19(7), 2242-2251 (2011-03-23)
The quinoline nucleus of the previously described 4-phenylquinoline-3-carboxamides NK(1) receptor ligands 7 has been transformed into either substituted or azole-(i.e., triazole or tetrazole) fused pyridine moieties of compounds 9 and 10, respectively, in order to obtain NK(1) receptor ligands showing
A D Vaz et al.
Drug metabolism and disposition: the biological fate of chemicals, 20(1), 108-112 (1992-01-01)
The binding to human placental aromatase cytochrome P-450 and resulting extent of inhibition was examined for pyridine, pyridines substituted at the 2-, 3-, or 4-positions with phenyl or benzoyl groups, and the nonpyridinic structural analogs biphenyl and benzophenone. Spectral binding
M R Gluck et al.
The Journal of biological chemistry, 269(5), 3167-3174 (1994-02-04)
We have investigated the mechanism of the inhibition of membrane-bound NADH dehydrogenase by 1-methyl-4-phenylpyridinium (MPP+) and a series of its 4'-alkyl-substituted analogs of increasing hydrophobicity, as well as their neutral, desmethyl congeners. Comparison of hydrophobicity, as measured by partition coefficients
C C Huang et al.
Brain research, 753(1), 27-35 (1997-04-04)
The effects of L-deprenyl (selegiline), a highly selective monoamine oxidase type B (MAO-B) inhibitor, on cell excitability of rat hippocampal CA1 neurons were examined in slice preparations using intracellular recording techniques. Superfusion of L-deprenyl (10 and 20 microM) reversibly limited
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service