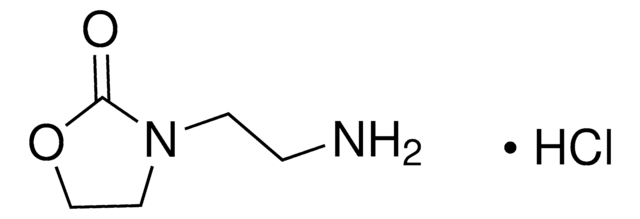
O9409
2-Oxazolidinone
98%
Synonym(s):
1,3-Oxazolidin-2-one, 2-Oxazolidone, 2-Oxo-1,3-oxazolidine, 2-Oxotetrahydro-1,3-oxazole
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C3H5NO2
CAS Number:
Molecular Weight:
87.08
Beilstein:
106251
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
220 °C/48 mmHg (lit.)
mp
83-87 °C (lit.)
SMILES string
O=C1NCCO1
InChI
1S/C3H5NO2/c5-3-4-1-2-6-3/h1-2H2,(H,4,5)
InChI key
IZXIZTKNFFYFOF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Oxazolidinone can be employed as a precursor for the preparation of acryloyloxazolidin-2-ones , trans-3-cinnamoyloxazolidin-2-one, 3-bromo-2-oxazolidinone , 4-methoxy-2-oxazolidinone , coordination compound, tetrakis[μ-(2-oxazolidinonato-κN3:κO2)]tetra-, (4Cu-Cu).
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Cysteine-Aminoethylation-Assisted Chemical Ubiquitination of Recombinant Histones
Chu G-C, et al.
Journal of the American Chemical Society, 141(8), 3654-3663 (2019)
Photoinduced, copper-catalyzed alkylation of amides with unactivated secondary alkyl halides at room temperature
Do Hien-Quang, et al.
Journal of the American Chemical Society, 136(5), 2162-2167 (2014)
Arun Mattappalil et al.
Clinical therapeutics, 36(11), 1489-1511 (2014-12-03)
Mild adverse drug reactions typically associated with antimicrobials are familiar to most clinicians. However, rare phenomena, such as neurotoxicity, are often unpredictable and potentially unexpected. The toxic effects of antimicrobials on the central nervous system are often underreported and the
Asymmetric Diels-Alder Reaction of α,β -Unsaturated Oxazolidin-2-one Derivatives Catalyzed by a Chiral Fe (III)-Bipyridine Diol Complex
Li Mao, et al.
Organic Letters, 20(4), 995-998 (2018)
Stereocontrolled Synthesis of trans/cis-2, 3-Disubstituted Cyclopropane-1, 1-diesters and Applications in the Syntheses of Furanolignans
Shen Y, et al.
The Journal of Organic Chemistry, 83(20), 12549-12558 (2018)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service