All Photos(1)
About This Item
Empirical Formula (Hill Notation):
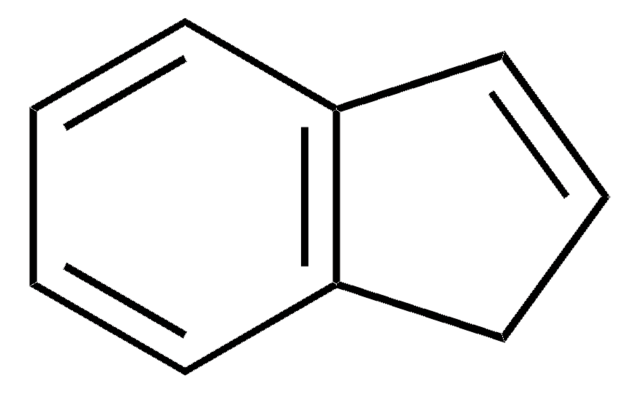
C9H10
CAS Number:
Molecular Weight:
118.18
Beilstein:
1904376
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.537 (lit.)
bp
176 °C (lit.)
mp
−51 °C (lit.)
density
0.965 g/mL at 25 °C (lit.)
SMILES string
C1Cc2ccccc2C1
InChI
1S/C9H10/c1-2-5-9-7-3-6-8(9)4-1/h1-2,4-5H,3,6-7H2
InChI key
PQNFLJBBNBOBRQ-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
129.2 °F - closed cup
Flash Point(C)
54 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Konstantin Ulanenko et al.
The Journal of organic chemistry, 71(18), 7053-7056 (2006-08-26)
5-Dimethylamino-1-aminoindan undergoes thermal decomposition and reacts with 6-chlorouracil to give 5-indanyl-6-chlorouracil derivative 9. The formation of 9 may be rationalized by a putative mechanism based on the intermediacy of the imminium methide species 8a.
Christopher S Frampton et al.
Acta crystallographica. Section C, Crystal structure communications, 68(Pt 8), o323-o326 (2012-08-02)
The title molecular salt, C(8)H(12)N(+)·C(26)H(21)O(3)(-), contains a dimeric indane pharmacophore that demonstrates potent anti-inflammatory activity. The indane group of the anion exhibits some disorder about the α-C atom, which appears common to many structures containing this group. A model to
Yuka Kobayashi et al.
Chirality, 17(2), 108-112 (2005-01-22)
Both novel enantiopure trans-1-aminobenz[f]indan-2-ols (4) were obtained from the racemate by the diastereomeric salt formation with (+)- and (-)-dibenzoyltartaric acids (8), respectively, and the absolute configuration of the enantiomer 4 in the less-soluble diastereomeric salt of racemic 4 with (+)-8
Ju-Ok Lim et al.
European journal of medicinal chemistry, 44(1), 322-331 (2008-04-15)
A series of bicyclic analogues having indan and tetrahydronaphthalene templates in the A-region were designed as conformationally constrained analogues of our previously reported potent TRPV1 antagonists (1, 3). The activities for rat TRPV1 of the conformationally restricted analogues were moderately
Mónica López-García et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 10(12), 3006-3014 (2004-06-24)
The efficient chemoenzymatic synthesis of enantiopure 1,3-difunctionalized indane derivatives has been achieved. The corresponding cis and trans N-protected amino alcohols were successfully resolved by acetylation using lipase B, which is a biocatalyst isolated from Candida antarctica. All the possible isomers
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service