H17082
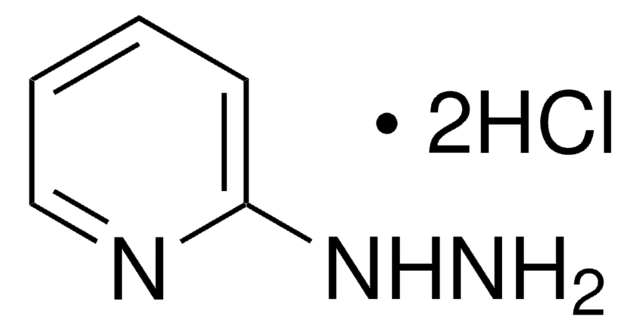
2-Hydrazinopyridine
97%
Synonym(s):
2-Pyridylhydrazine
About This Item
Recommended Products
Quality Level
Assay
97%
bp
90-92 °C/1 mmHg (lit.)
mp
41-44 °C (lit.)
storage temp.
2-8°C
SMILES string
NNc1ccccn1
InChI
1S/C5H7N3/c6-8-5-3-1-2-4-7-5/h1-4H,6H2,(H,7,8)
InChI key
NWELCUKYUCBVKK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Enhanced Metal Removal: Utilizing Schiff base functionalized dialdehyde starch, derived from 2-hydrazinopyridine, demonstrated significant potential in enhancing the removal of Cu(II) from solutions. The study included preparation methodologies, performance evaluations, and DFT calculations, showcasing its efficacy in water treatment technologies (Liang et al., 2024).
- Dual Sensing Probe Development: A novel dicyanisophorone-based probe, incorporating 2-hydrazinopyridine, was developed for the dual sensing of Zn(2+) and Cd(2+) via near-infrared fluorescence. This advancement aids in the detection and analysis of heavy metals in various environmental and biological samples (Yan et al., 2023).
- Active Site Analysis in Lysyl Oxidase: The study provided insights into the spatial arrangement of active site components in Lysyl Oxidase-like 2, including the role of 2-hydrazinopyridine, which is critical for understanding the enzyme′s mechanism and potential therapeutic applications (Meier et al., 2022).
- Structural Analysis of Lysyl Oxidase: Research focused on the predicted 3D structure of the amine oxidase domain of Lysyl Oxidase-Like 2, exploring the interaction dynamics facilitated by 2-hydrazinopyridine. This contributes significantly to the field of molecular biology and enzyme function analysis (Meier et al., 2022).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service