E33904
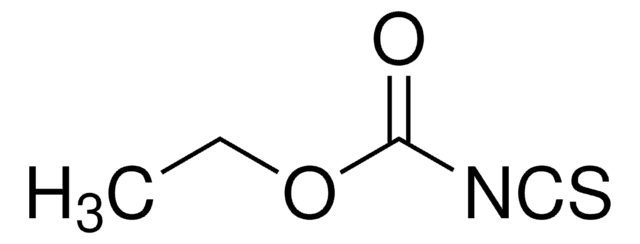
Ethyl isothiocyanate
97%
Synonym(s):
Ethyl mustard oil
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C2H5NCS
CAS Number:
Molecular Weight:
87.14
Beilstein:
1737705
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.512 (lit.)
bp
130-132 °C (lit.)
mp
−6 °C (lit.)
density
0.995 g/mL at 25 °C (lit.)
SMILES string
CCN=C=S
InChI
1S/C3H5NS/c1-2-4-3-5/h2H2,1H3
InChI key
HBNYJWAFDZLWRS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Flam. Liq. 3 - Resp. Sens. 1 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
75.2 °F - closed cup
Flash Point(C)
24 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hirokuni Tajima et al.
Bioscience, biotechnology, and biochemistry, 67(8), 1844-1846 (2003-09-03)
Hydroxy isothiocyanates, especially 2-(4-hydroxyphenyl)ethyl isothiocyanate (hITC), were examined for antimicrobial synergism with streptomycin (SM) against Escherichia coli. On the course of those experiments, a peculiar suppression of SM by a low concentration of hITC was observed, besides the antibacterial synergism
Antonietta Melchini et al.
Journal of medicinal chemistry, 55(22), 9682-9692 (2012-09-25)
Dietary isothiocyanates (ITC) derived from cruciferous vegetables have been shown to have numerous biological effects consistent with chemoprotective activity. In this study we synthesized a novel ITC, 2-(2-pyridyl) ethyl ITC (PY-ITC), and assessed its chemopreventive ability in comparison to sulforaphane
W H Mennicke et al.
Xenobiotica; the fate of foreign compounds in biological systems, 13(4), 203-207 (1983-04-01)
The metabolism of methyl, ethyl, butyl and allyl isothiocyanate, which occur as glucosinolates in a number of plants, was studied. Oral administration of the substances to the rat was followed by their renal excretion as mercapturic acids, which were isolated
James R Durig et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 68(3), 783-795 (2007-04-17)
Variable temperature (-105 to -150 degrees C) studies of the infrared spectra (3500-400 cm(-1)) of ethylisothiocyanate, CH(3)CH(2)NCS, dissolved in liquid krypton have been recorded. Additionally the infrared spectra of the gas and solid have been re-investigated. These spectroscopic data indicate
Juyoung Lee et al.
Journal of the science of food and agriculture, 95(11), 2244-2251 (2014-10-02)
Glucosinolates are abundant in Brassicaceae vegetables, and they are degraded into various organic breakdown products (BPs) (R-CN, -NCS and -SCN) by myrosinase when plant tissues are damaged. This study was designed to investigate whether these BPs could be broken further
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service