D124001
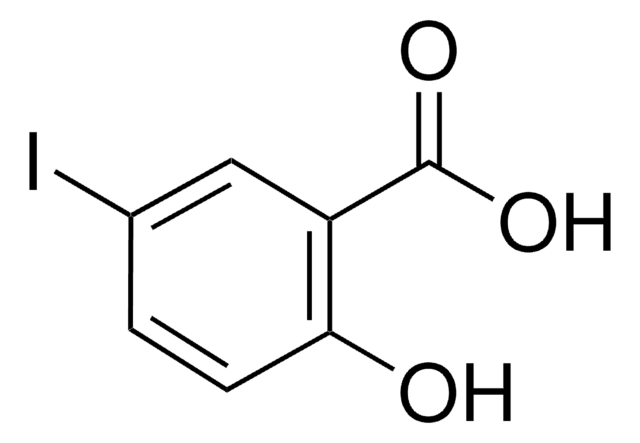
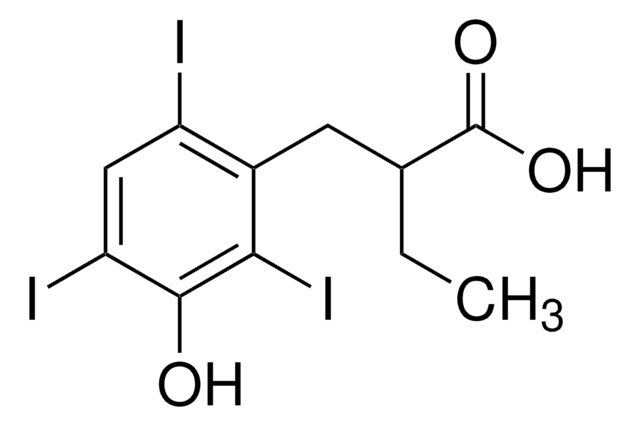
3,5-Diiodosalicylic acid
99%
Synonym(s):
2-Hydroxy-3,5-diiodobenzoic acid, 3,5-Diiodo-2-hydroxybenzoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
I2C6H2(OH)CO2H
CAS Number:
Molecular Weight:
389.91
Beilstein:
2615358
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
mp
220-230 °C (dec.) (lit.)
SMILES string
OC(=O)c1cc(I)cc(I)c1O
InChI
1S/C7H4I2O3/c8-3-1-4(7(11)12)6(10)5(9)2-3/h1-2,10H,(H,11,12)
InChI key
DHZVWQPHNWDCFS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
D Restrepo et al.
The Journal of general physiology, 100(5), 825-846 (1992-11-01)
We have developed a new test to differentiate between ping-pong and simultaneous mechanisms for tightly coupled anion exchange. This test requires the use of a dead-end reversible noncompetitive inhibitor. As an example, we have applied the test to the anion
L Simchowitz et al.
The American journal of physiology, 261(5 Pt 1), C906-C915 (1991-11-01)
Organotin derivatives represent a class of artificial ionophores that mediate Cl(-)-OH- exchange and thereby facilitate the chemical equilibrium distribution of Cl- and H+ across biological membranes. Imposing different pH and Cl- gradients by varying extracellular pH (pHo) and extracellular [Cl-]
D Riendeau et al.
Molecular and cellular biochemistry, 71(1), 45-52 (1986-06-01)
Lithium diiodosalicylate (LIS) was used to selectively solubilize proteins from purified intestinal brush border membrane vesicles. Incubation of the vesicles with increasing concentrations of LIS resulted in the progressive release of proteins with total disruption of the membranes being obtained
Nuclear frameworks: concepts and operational definitions.
N Stuurman et al.
Cell biology international reports, 16(8), 837-852 (1992-08-01)
P Belgrader et al.
Journal of cell science, 98 ( Pt 3), 281-291 (1991-03-01)
Different agents have been employed to extract the histones and other soluble components from isolated HeLa S3 nuclei during nuclear matrix isolation. We report that 0.2M (NH4)2SO4 is a milder extracting agent than NaCl and LIS (lithium 3,5-diiodosalicylate), on the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service