All Photos(1)
About This Item
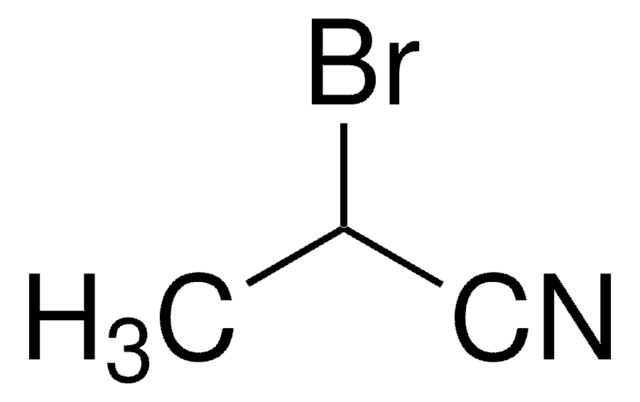
Linear Formula:
Br(CH2)4CN
CAS Number:
Molecular Weight:
162.03
Beilstein:
1742080
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.478 (lit.)
density
1.388 g/mL at 25 °C (lit.)
SMILES string
BrCCCCC#N
InChI
1S/C5H8BrN/c6-4-2-1-3-5-7/h1-4H2
InChI key
NWWWGAKVHCSAEU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
5-Bromovaleronitrile can be used as a reactant to prepare:
- A key intermediate benzyl(methyl)amino)pentanenitrile, which is used in the synthesis of N-methylcadaverine.
- 1-cyanobutyl-3-alkylbenzimidazolium bromide salt by reacting with various N-alkylbenzimidazoles.
- Tridecanenitrile by Ni-catalyzed cross-coupling reaction with dioctylzinc in the presence of tetraene and MgBr2.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Kayla N Anderson et al.
Molecules (Basel, Switzerland), 23(5) (2018-05-23)
Alkaloids compose a large class of natural products, and mono-methylated polyamines are a common intermediate in their biosynthesis. In order to evaluate the role of selectively methylated natural products, synthetic strategies are needed to prepare them. Here, N-methylcadaverine is prepared
Nitrile functionalized silver (I) N-heterocyclic carbene complexes: DFT calculations and antitumor studies
Hussaini SY, et al.
Transition Metal Chemistry, 43(4), 301-312 (2018)
Michelle Muñoz-Osses et al.
Dalton transactions (Cambridge, England : 2003), 47(4), 1233-1242 (2018-01-05)
Substituted amino-piperazine derivatives were synthesized and used as precursors for the preparation of a series of new organometallic Re(i) imine complexes with the general formula [(η
Nickel-catalyzed cross-coupling reaction of alkyl halides with organozinc and Grignard reagents with 1,3,8,10-tetraenes as additives.
Jun Terao et al.
Angewandte Chemie (International ed. in English), 43(45), 6180-6182 (2004-11-19)
Franziska Kupke et al.
Scientific reports, 6, 37631-37631 (2016-11-25)
Isothiocyanates are the most intensively studied breakdown products of glucosinolates from Brassica plants and well recognized for their pleiotropic effects against cancer but also for their genotoxic potential. However, knowledge about the bioactivity of glucosinolate-borne nitriles in foods is very
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service