914088
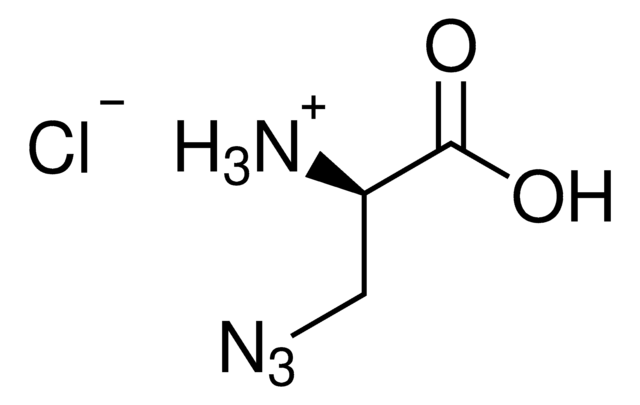
N6-((2-Azidoethoxy)carbonyl)-L-lysine hydrochloride
≥95%
Synonym(s):
(S)-2-amino-6-((2-azidoethoxy)carbonylamino)hexanoic acid hydrochloride, Clickable amino acid for bioconjugation, H-L-Lys(EO-N3)-OH HCl, Lysine-azide, UAA crosslinker
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H17N5O4 · xHCl
CAS Number:
Molecular Weight:
259.26 (free base basis)
UNSPSC Code:
12352209
Recommended Products
Application
N6-((2-Azidoethoxy)carbonyl)-L-lysine hydrochloride is a clickable amino acid derivative for site-specific incorporation into recombinant proteins or synthesis of chemical probes and tools for biological applications. This non-canonical lysine possesses an azide for bioorthogonal reaction with alkynes.
Other Notes
Construction of bacterial cells with an active transport system for unnatural amino acids
Semisynthesis of an Active Enzyme by Quantitative Click Ligation
A Robust and Quantitative Reporter System To Evaluate Noncanonical Amino Acid Incorporation in Yeast
An orthogonalized platform for genetic code expansion in both bacteria and eukaryotes
Semisynthesis of an Active Enzyme by Quantitative Click Ligation
A Robust and Quantitative Reporter System To Evaluate Noncanonical Amino Acid Incorporation in Yeast
An orthogonalized platform for genetic code expansion in both bacteria and eukaryotes
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Self-react. C
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Abhishek Chatterjee et al.
Biochemistry, 52(10), 1828-1837 (2013-02-06)
To site-specifically incorporate an unnatural amino acid (UAA) into target proteins in Escherichia coli, we use a suppressor plasmid that provides an engineered suppressor tRNA and an aminoacyl-tRNA synthetase (aaRS) specific for the UAA of interest. The continuous drive to
Duy P Nguyen et al.
Journal of the American Chemical Society, 131(25), 8720-8721 (2009-06-12)
We demonstrate that an orthogonal Methanosarcina barkeri MS pyrrolysyl-tRNA synthetase/tRNA(CUA) pair directs the efficient, site-specific incorporation of N6-[(2-propynyloxy)carbonyl]-L-lysine, containing a carbon-carbon triple bond, and N6-[(2-azidoethoxy)carbonyl]-L-lysine, containing an azido group, into recombinant proteins in Escherichia coli. Proteins containing the alkyne functional
Sigrid Milles et al.
Journal of the American Chemical Society, 134(11), 5187-5195 (2012-02-24)
Single-molecule methods have matured into central tools for studies in biology. Foerster resonance energy transfer (FRET) techniques, in particular, have been widely applied to study biomolecular structure and dynamics. The major bottleneck for a facile and general application of these
Ralph E Kleiner et al.
Journal of the American Chemical Society, 135(34), 12520-12523 (2013-08-13)
Microtubules are hollow tube-like biological polymers required for transport in diverse cellular contexts and are important drug targets. Microtubule function depends on interactions with associated proteins and post-translational modifications at specific sites located on its interior and exterior surfaces. However
Christian Kofoed et al.
Bioconjugate chemistry, 30(4), 1169-1174 (2019-03-19)
The incorporation of clickable noncanonical amino acids (ncAAs) has proven to an invaluable tool in chemical biology and protein science research. Nevertheless, the number of examples in which the method is used for preparative purposes is extremely limited. We report
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service