90303
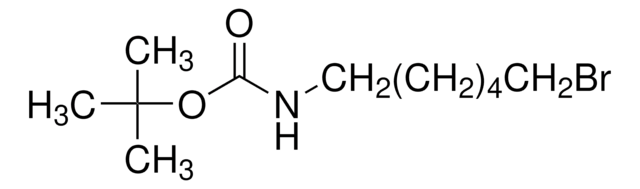
4-(Boc-amino)butyl bromide
technical, ≥90% (AT)
Synonym(s):
Carbamic acid, (4-bromobutyl)-, 1,1-dimethylethyl ester, tert-Butyl N-(4-bromobutyl)carbamate
About This Item
Recommended Products
grade
technical
Quality Level
Assay
≥90% (AT)
form
liquid
reaction suitability
reagent type: cross-linking reagent
impurities
~5% 1-boc-pyrrolidine
functional group
Boc
amine
bromo
storage temp.
2-8°C
SMILES string
BrCCCCNC(OC(C)(C)C)=O
InChI
1S/C9H18BrNO2/c1-9(2,3)13-8(12)11-7-5-4-6-10/h4-7H2,1-3H3,(H,11,12)
1 of 4
This Item | 100919 | 15404 | 15302 |
|---|---|---|---|
| Quality Level 100 | Quality Level 200 | Quality Level 100 | Quality Level 100 |
| reaction suitability reagent type: cross-linking reagent | reaction suitability reagent type: cross-linking reagent, reagent type: linker | reaction suitability reagent type: cross-linking reagent | reaction suitability reagent type: cross-linking reagent |
| storage temp. 2-8°C | storage temp. - | storage temp. - | storage temp. 2-8°C |
| form liquid | form - | form - | form - |
| grade technical | grade - | grade - | grade - |
Application
- For the synthesis of N-Boc-aminoalkoxyphenyl derivatives, precursor to pharmacophore elements for the treatment of glaucoma.[1]
- For the synthesis of various aloperine derivatives with potential application as anti-HIV agents.[2]
- For the modification of 4,5,6,7-tetrabromobenzotriazole (TBB) derivatives to generate improved CK2 inhibitors.[3]
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service