705055
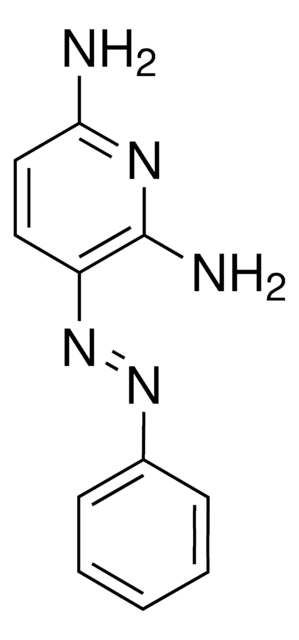
4,4′-Azopyridine
Synonym(s):
Azobis(4-pyridine)
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H8N4
CAS Number:
Molecular Weight:
184.20
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
Quality Level
mp
96-101 °C
SMILES string
c1cc(ccn1)N=Nc2ccncc2
InChI
1S/C10H8N4/c1-5-11-6-2-9(1)13-14-10-3-7-12-8-4-10/h1-8H
InChI key
XUPMSLUFFIXCDA-UHFFFAOYSA-N
General description
4,4′-Azopyridine is an aromatic heterocyclic compound used as a ligand in the formation of [HgI2(4,4′-azopyridine)]n complex.
Application
4,4′-Azopyridine can be used:
- To prepare porous coordination polymers (PCPs) by reacting with Zn(NO3)2 and 1,4-benzenedicarboxylic acid.
- As a reagent for the conversion of aliphatic alcohols into disulfides under Mitsunobu conditions.
- To prepare 4,4′-azopyridine-bridged binuclear zinc(II) complexes.
Reactant for preparation of:
Reagent in:
- Flexible porous coordination polymers constructed from 1,2-bis(4-pyridyl)hydrazine via solvothermal in situ reduction reaction
- Metal-organic frameworks based on transition-metal carboxylate clusters as secondary building units
- Heteroaromatic azo compounds under Mitsunobu conditions
- MnII, CoII, and ZnII coordination polymers
- Low-dimensional and porous coordination compounds capable of supramolecular aromatic interactions
Reagent in:
- Facile Mitsunobu esterification reactions
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A novel 4, 4′-azopyridine-bridged binuclear zinc complex
Zhu L, et al.
Journal of Coordination Chemistry, 56(17), 1447-1453 (2003)
A novel HgI2 adduct with an azopyridine ligand: synthesis, structure and optical refractive effect of [HgI2 (4, 4?-azopyridine)] n
Niu, Yunyin and Song
CrystEngComm, 3, 152-154 (2001)
Flexible porous coordination polymers constructed from 1, 2-bis (4-pyridyl) hydrazine via solvothermal in situ reduction of 4, 4′-azopyridine
Liu X, et al.
Dalton Transactions, 40(34), 8549-8554 (2011)
Heteroaromatic azo compounds as efficient and recyclable reagents for direct conversion of aliphatic alcohols into symmetrical disulfides
Iranpoor N, et al.
Tetrahedron Letters, 53(51), 6913-6915 (2012)
4, 4?-Azopyridine as an easily prepared and recyclable oxidant for synthesis of symmetrical disulfides from thiols or alkyl halides (tosylates)/thiourea}
Khalili, Dariush and Iranpoor
Journal of Sulfur Chemistry, 36, 544-555 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service