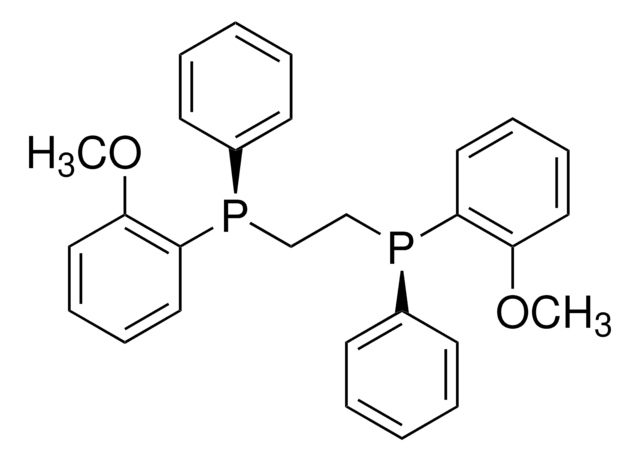
676403
(R,R)-(–)-2,3-Bis(tert-butylmethylphosphino)quinoxaline
≥95%
Synonym(s):
(R) QuinoxP®
About This Item
Recommended Products
Assay
≥95%
form
solid
mp
100-104 °C
functional group
phosphine
SMILES string
CP(c1nc2ccccc2nc1P(C)C(C)(C)C)C(C)(C)C
InChI
1S/C18H28N2P2/c1-17(2,3)21(7)15-16(22(8)18(4,5)6)20-14-12-10-9-11-13(14)19-15/h9-12H,1-8H3/t21-,22-/m0/s1
InChI key
DRZBLHZZDMCPGX-VXKWHMMOSA-N
Related Categories
General description
Application
Features and Benefits
- It is not oxidized nor epimerized at ambient conditions in air
- Enantioselectivities are outstanding for various reaction paradigms
- Hydrogenations proceed under mild reaction conditions
- Low catalyst loadings yield high TONs
Citation
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 4 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
QuinoxP*: Air-Stable and Highly Efficient and Productive Chiral Ligands
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![(R)-1-[(SP)-2-(Diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphine ≥97%](/deepweb/assets/sigmaaldrich/product/structures/245/493/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0/640/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0.png)
![(+)-1,2-Bis[(2S,5S)-2,5-dimethylphospholano]benzene kanata purity](/deepweb/assets/sigmaaldrich/product/structures/319/912/cec7b70f-bf7c-4a96-9f11-a73ae892e34c/640/cec7b70f-bf7c-4a96-9f11-a73ae892e34c.png)