633348
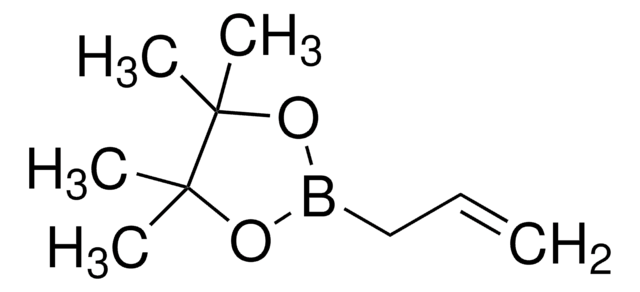
Vinylboronic acid pinacol ester
contains phenothiazine as stabilizer, 95%
Synonym(s):
2-Ethenyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, 2-Vinyl-4,4,5,5-tetramethyl-1,3,2-dioxaoborolane, 4,4,5,5-Tetramethyl-2-vinyl-1,3,2-dioxaborolane
About This Item
Recommended Products
Quality Level
Assay
95%
contains
phenothiazine as stabilizer
refractive index
n20/D 1.4300 (lit.)
density
0.908 g/mL at 25 °C (lit.)
storage temp.
−20°C
SMILES string
CC1(C)OB(OC1(C)C)C=C
InChI
1S/C8H15BO2/c1-6-9-10-7(2,3)8(4,5)11-9/h6H,1H2,2-5H3
InChI key
DPGSPRJLAZGUBQ-UHFFFAOYSA-N
Application
- Suzuki-Miyaura coupling reactions
- Mizoroki-Heck reactions (cascade reaction)
- Intramolecular Nozaki-Hiyama-Kishi reactions
- Stereoselective Cu-catalyzed γ-selective and stereospecific coupling
- Control of stereoselectivity and mechanistic portrait on intramolecular (4+1)-cycloaddition of dialkoxycarbenes
- Regio- and stereoselective synthesis of trisubstituted alkenes via gold(I)-catalyzed hydrophosphoryloxylation of haloalkynes followed by Pd-catalyzed consecutive cross-coupling reactions
- Asymmetric Birch reductive alkylation
Reagent used in Preparation of
- Molecular tubes for lipid sensing
- Enzymatic inhibitors, antibiotics, receptor analogs, and other biologically significant compounds (including total syntheses)
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Flam. Liq. 3 - Skin Sens. 1
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
93.2 °F
Flash Point(C)
34 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)