All Photos(1)
About This Item
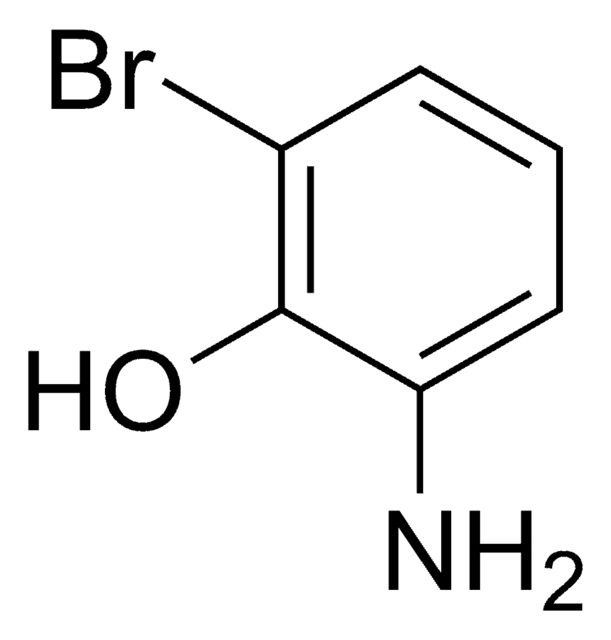
Linear Formula:
H2NC6H3(Cl)OH
CAS Number:
Molecular Weight:
143.57
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
145-153 °C (lit.)
functional group
chloro
SMILES string
Nc1ccc(Cl)cc1O
InChI
1S/C6H6ClNO/c7-4-1-2-5(8)6(9)3-4/h1-3,9H,8H2
InChI key
FZCQMIRJCGWWCL-UHFFFAOYSA-N
General description
2-Amino-5-chlorophenol can be synthesized from 2-chloro-5-nitrophenol via reduction. It can also be obtained from 1-chloro-4-nitrobenzene by using a bacterial strain LW1. 2-Amino-5-chlorophenol participates in the condensation reaction with acetylferrocene to afford ferrocenyl Schiff bases bearing a phenol group.
Application
2-Amino-5-chlorophenol may be used to synthesize 2-amino-5-chloromuconic semialdehyde and benzoxazole derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jian-Feng Wu et al.
Archives of microbiology, 183(1), 1-8 (2004-12-08)
Comamonas strain CNB-1 was isolated from a biological reactor treating wastewater from a p-chloronitrobenzene production factory. Strain CNB-1 used p-chloronitrobenzene as sole source of carbon, nitrogen, and energy. A 2-aminophenol 1,6-dioxygenase was purified from cells of strain CNB-1. The purified
Synthesis and Crystal Structure of 1-Chloro-2-methyl-4-nitrobenzene.
Saeed A and Simpson J.
Crystals, 2(1), 137-143 (2012)
M Valentovic et al.
Toxicology and applied pharmacology, 161(1), 1-9 (1999-11-24)
2-Amino-5-chlorophenol is nephrotoxic through an unidentified mechanism. This study examined the in vitro toxicity of 2-amino-5-chlorophenol in renal cortical slices from Fischer 344 rats and specifically assessed induction of lipid peroxidation and depletion of renal glutathione. Renal cortical slices exposed
Yi Xiao et al.
Applied microbiology and biotechnology, 73(1), 166-171 (2006-04-28)
The genes encoding enzymes involved in the initial reactions during degradation of 4-chloronitrobenzene (4CNB) were characterized from the 4CNB utilizer Pseudomonas putida ZWL73, in which a partial reductive pathway was adopted. A DNA fragment containing genes coding for chloronitrobenzene nitroreductase
E Katsivela et al.
Applied and environmental microbiology, 65(4), 1405-1412 (1999-04-02)
Bacterial strain LW1, which belongs to the family Comamonadaceae, utilizes 1-chloro-4-nitrobenzene (1C4NB) as a sole source of carbon, nitrogen, and energy. Suspensions of 1C4NB-grown cells removed 1C4NB from culture fluids, and there was a concomitant release of ammonia and chloride.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service