550019
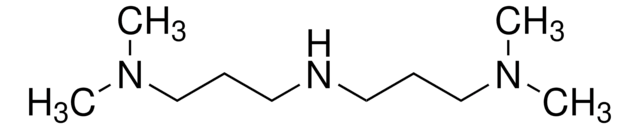
N,N-Dimethyldipropylenetriamine
99%
Synonym(s):
DMAPAPA
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)2N(CH2)3NH(CH2)3NH
CAS Number:
Molecular Weight:
159.27
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
refractive index
n20/D 1.4630 (lit.)
bp
220 °C (lit.)
density
0.883 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
CN(C)CCCNCCCN
InChI
1S/C8H21N3/c1-11(2)8-4-7-10-6-3-5-9/h10H,3-9H2,1-2H3
InChI key
OMKZWUPRGQMQJC-UHFFFAOYSA-N
General description
N,N-Dimethyldipropylenetriamine is an aliphatic acyclic amine that is commonly used to develop polymeric system for gene delivery.
Application
N,N-Dimethyldipropylenetriamine (DP) may be used to:
- Prepare poly(ethylene oxide)-b-poly(3-caprolactone-g-DP) [PEO-b-P(CL-g-DP)], a biodegradable amphiphilic polycationic copolymer.
- Develop a second generation aminoglycoside 6′-N-acetyltransferase (AAC(6′) inhibitor.
- Prepare a hydrophilic gemcitabine conjugated cationic copolymer, which can be employed in the treatment pancreatic cancer.
Legal Information
Product of Arkema Inc.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 2 - Skin Corr. 1A - Skin Sens. 1
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
210.2 °F - closed cup
Flash Point(C)
99 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Anupama Mittal et al.
Biomaterials, 35(25), 7077-7087 (2014-05-20)
Clinical effectiveness of gemcitabine in pancreatic cancer is hindered due to its rapid plasma metabolism and development of chemo-resistance. We have previously delineated the significant role of miRNAs in mediating the growth and proliferation of cancer stem cells (CSCs) which
Feng Gao et al.
Journal of medicinal chemistry, 49(17), 5273-5281 (2006-08-18)
Truncated aminoglycoside-coenzyme A bisubstrate analogues were efficiently prepared using a convergent approach where the amine and the thiol are coupled in one pot with the addition of a linker, without the need for protecting groups. These derivatives were tested for
Xiao-Bing Xiong et al.
Biomaterials, 30(2), 242-253 (2008-10-08)
The RNA interference (RNAi) technology has been successfully used in elucidating mechanisms behind various biological events. However, in the absence of safe and effective carriers for in vivo delivery of small interfering RNAs (siRNAs), application of this technology for therapeutic
[Toxicity of several aliphatic amines].
G I Sidorin et al.
Gigiena truda i professional'nye zabolevaniia, (11)(11), 50-53 (1984-11-01)
J Shen et al.
Biomaterials, 34(18), 4520-4531 (2013-03-23)
The low toxicity and efficient gene delivery of polymeric vectors remain the major barrier to the clinical application of non-viral gene therapy. Here, we present a poly-D, L-succinimide (PSI)-based biodegradable cationic polymer which mimicked the golden standard, branched polyethylenimine (PEI
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service