54920
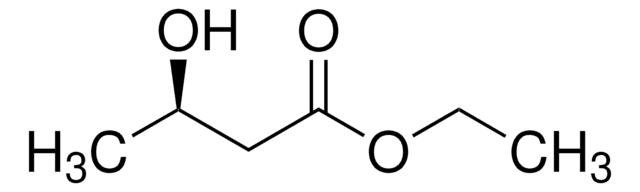
(R)-3-Hydroxybutyric acid
≥98.0% (T)
Synonym(s):
(3R)-3-Hydroxybutanoic acid, (3R)-Hydroxybutyrate, 3R-Hydroxybutanoic acid, D-β-Hydroxybutyrate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H8O3
CAS Number:
Molecular Weight:
104.10
Beilstein:
1720568
EC Number:
MDL number:
UNSPSC Code:
51113400
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (T)
form
solid
optical activity
[α]20/D −25±1°, c = 6% in H2O
mp
49-50 °C (lit.)
functional group
carboxylic acid
hydroxyl
storage temp.
2-8°C
SMILES string
C[C@@H](O)CC(O)=O
InChI
1S/C4H8O3/c1-3(5)2-4(6)7/h3,5H,2H2,1H3,(H,6,7)/t3-/m1/s1
InChI key
WHBMMWSBFZVSSR-GSVOUGTGSA-N
Looking for similar products? Visit Product Comparison Guide
Application
(R)-3-Hydroxybutyric acid may be used in the preparation of copolymers by reacting with various hydroxyalkanoic acids.1 It may also be used in the preparation of (3R,4R)-4-acetoxy-3-[(R)-1-(formyloxy)ethyl]-2-azetidinone.2
Other Notes
Important chiral starting material; enantioselective reactions at the 2-, 3- and 4-positions via the cyclic acetal with aldehydes; preparation of (R)-β-butyrolactone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
D. Seebach et al.
Modern Synthetic Methods, 4, 125-125 (1986)
Journal of the American Chemical Society, 110, 4763-4763 (1988)
A. Griesbech et al.
Helvetica Chimica Acta, 70, 1320-1320 (1987)
J L Hansen et al.
Clinical chemistry, 24(3), 475-479 (1978-03-01)
Methods are described for direct assays of lactate, pyruvate, beta-hydroxybutyrate, and acetoacetate in plasma with the GEMSAEC centrifugal analyzer. The methods for lactate, beta-hydroxybutyrate, and acetoacetate are kinetic and ratiometric, eliminating the need for specimen-blank assays. The pyruvate method is
Sodium-glucose cotransporter 2 inhibitors regulate ketone body metabolism via inter-organ crosstalk.
Jin Hee Kim et al.
Diabetes, obesity & metabolism (2018-11-09)
To investigate sodium-glucose cotransporter 2 inhibitor (SGLT2i)-induced changes in ketogenic enzymes and transporters in normal and diabetic mice models. Normal mice were randomly assigned to receive either vehicle or SGLT2i (25 mg/kg/d by oral gavage) for 7 days. Diabetic mice were treated
Chromatograms
suitable for GCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![Poly[(R)-3-hydroxybutyric acid] natural origin](/deepweb/assets/sigmaaldrich/product/structures/129/476/7d1c924b-f644-4889-a2d6-d7a923ce382c/640/7d1c924b-f644-4889-a2d6-d7a923ce382c.png)