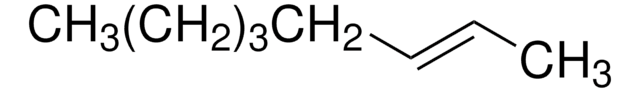538493
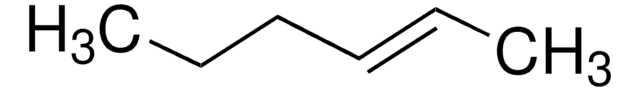
cis-2-Hexene
95%
Synonym(s):
(2Z)-2-Hexene, (Z)-2-Hexene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CH2CH2CH=CHCH3
CAS Number:
Molecular Weight:
84.16
Beilstein:
1719019
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
refractive index
n20/D 1.396 (lit.)
bp
68-70 °C (lit.)
density
0.669 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CCC\C=C/C
InChI
1S/C6H12/c1-3-5-6-4-2/h3,5H,4,6H2,1-2H3/b5-3-
InChI key
RYPKRALMXUUNKS-HYXAFXHYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
cis-2-Hexene is one of the isomeric forms of hexene. It is obtained from 2-hexyne, via semihydrogenation in the presence of Pd78(phen) [phen = 1,10-Phenanthroline] on titanium dioxide in n-octane. cis-2-Hexene is formed as one of the primary product from the dimerization of propylene in the presence of a nickel oxide-silica-alumina catalyst. It undergoes isomerization/hydrosilylation reaction with phenyl silane in the presence of cobalt catalyst to afford 1-hexylphenylsilane.
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-13.0 °F - closed cup
Flash Point(C)
-25 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rapid, Regioconvergent, Solvent-Free Alkene Hydrosilylation with a Cobalt Catalyst.
Chen C, et al.
Journal of the American Chemical Society, 137(41), 13244-13247 (2015)
Synthesis and catalytic properties of large ligand stabilized palladium clusters.
Schmid G, et al.
J. Mol. Catal. A: Chem., 107(1), 95-104 (1996)
The dimerization of propylene over a nickel oxide-silica-alumina catalyst.
Imai H, et al.
Bulletin of the Chemical Society of Japan, 41(1), 45-48 (1968)
Chiara Giorio et al.
Faraday discussions, 200, 559-578 (2017-06-06)
Ozonolysis of alkenes is a key reaction in the atmosphere, playing an important role in determining the oxidising capacity of the atmosphere and acting as a source of compounds that can contribute to local photochemical "smog". The reaction products of
Sören Reinhard et al.
ChemMedChem, 12(17), 1464-1470 (2017-07-18)
Cationic lipo-oligomers containing unsaturated oleic acid are potent siRNA carriers based on electrostatic and hydrophobic lipo-polyplex formation and endosomal membrane destabilization. Lipo-oligomers can be produced by solid-phase-supported synthesis in sequence-defined form. However, the trifluoroacetic acid (TFA)-mediated removal of acid-labile protecting
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service